Incidental finding of invasive lobular carcinoma within a borderline phyllodes tumour of the breast: a case report
Highlight box
Key findings
• The incidental finding of an invasive lobular carcinoma within a phyllodes tumour.
What is known and what is new?
• There is limited existing knowledge surrounding the coexistence of invasive carcinomas and Phyllodes Tumours.
• When these pathologies co-exist, the phyllodes tumours are typically of the malignant subtype and the carcinomas are DCIS, LCIS or IDC; not ILC as reported here.
• This case report characterizes the rare coexistence of invasive lobular carcinoma within a borderline phyllodes tumour.
What is the implication, and what should change now?
• The rarity of these two pathologies highlights the importance of management by an experienced multidisciplinary team on a case-by-case basis.
• This case seeks to emphasize the importance of thoroughly investigating breast lumps with discordant triple assessment findings under multidisciplinary team guidance.
Introduction
Phyllodes tumours (PTs), also known as cystosarcoma phyllodes, are rare neoplasms of the breast and comprise less than 1% of all breast neoplasms (1). Morphologically they represent a continuum and can be classified as ‘benign’, ‘borderline’ or ‘malignant’ based on several histological features (2,3). Around 10% of PTs are ‘malignant’ with these tumours showing a propensity for haematogenous rather than lymphatic spread (4,5). Whilst PTs are clinically and radiologically similar to fibroadenomas, they tend to occur more frequently in older women with a peak incidence at 45–49 years of age (6,7). The presence of invasive carcinoma within a PT is exceedingly rare, occurring in less than 1% of PTs (8,9). Where invasive carcinomas and PTs occur synchronously, invasive lobular carcinoma (ILC), rather than invasive ductal carcinoma (IDC), is rarer still (10).
We report a case of a woman in her early 40’s who underwent an excisional biopsy of a fibroadenoma where post-operative histology showed an incidental finding of a triple negative grade 2 ILC within a borderline PT. In addition to the importance of corroborating the scarce existing literature on the simultaneous presence of PTs and ILC, this case report crucially highlights the importance of rigorous investigative work-up and management under an experienced breast multidisciplinary team (MDT) to avoid missed diagnoses of breast cancers in seemingly benign breast lumps. We present the following case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-52/rc).
Case presentation
A 40-year-old woman presented to the one-stop breast clinic with a 2-month history of a lump in the right breast. This was associated with a 1-week history of associated discomfort and tightness which encouraged a visit to the General Practitioner where an urgent referral to the breast unit for specialist input was made. She had a past medical history of type 2 diabetes mellitus for which she was taking oral hypoglycaemics (metformin). She had no family history of breast or ovarian cancer. She was pre-menopausal with a regular menstrual cycle and was para 1.
On clinical examination, there was tender nodularity in the upper outer quadrant of the right breast as well as multiple suspected sebaceous cysts in the mid chest (P2/3). Based on these clinical findings she underwent an ultrasound scan of the right breast and a bilateral mammogram.
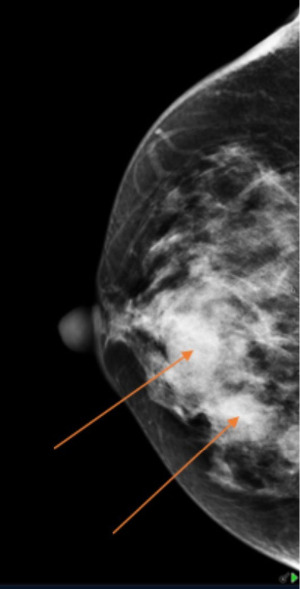
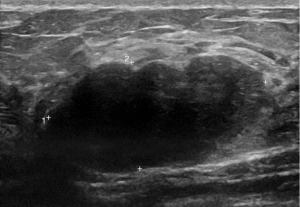
Mammography (Figure 1) showed dense fibroglandular appearances with lobulated densities (see arrows) in the upper outer quadrant of the right breast, possibly cysts, with few scattered microcalcifications suggestive of fibrocystic changes (RM3). Ultrasonography (Figure 2) of the symptomatic lump confirmed a lobulated hypoechoic nodule measuring 45 mm × 17 mm × 20 mm in the upper outer breast in keeping with a fibroadenoma (U3). Ultrasound-guided core biopsy of the lump was performed which reported histological features consistent with fibroadenoma (B2) and further immunohistochemistry with CK5 showed a benign staining pattern and no evidence of in situ or invasive malignancy.
This case was discussed in the Breast MDT meeting and, due to radiological findings suggestive of a PT and discordance on triple assessment, the consensus was to proceed with excisional biopsy of the lump.
At this stage, a diagnosis of probable fibroadenoma was made due to the clinical findings, core biopsy and ultrasonographic features of a lobulated hypoechoic mass. A PT was included in the differential diagnosis due to its known similarity in clinical and radiological features to fibroadenoma. The presence of microcalcifications on the mammogram also raised concerns as these features are not typically associated with a fibroadenoma.
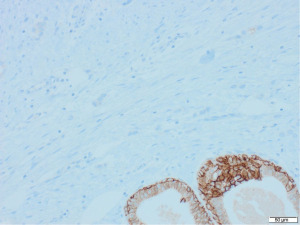
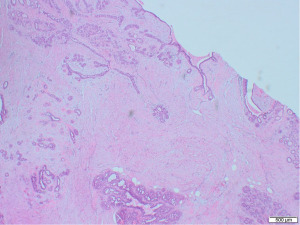
Excisional biopsy of right breast lump was performed under general anaesthesia and post-operative histology revealed an incidental ILC arising in a fibroepithelial lesion composed of both an ordinary fibroadenoma and a ‘borderline’ PT. The invasive component measured 6 mm with features consistent of a classical grade 2 ILC. The tumour was completely excised, all resection margins were clear by at least 4 mm and no lymphovascular invasion was seen. The tumour was considered triple negative with oestrogen receptor score 4/8, progesterone receptor score 0/8 and human epidermal growth factor receptor 2 (HER2). The Ki67 proliferation index was 3–5%. Microscopic appearance is shown in Figures 3-7.
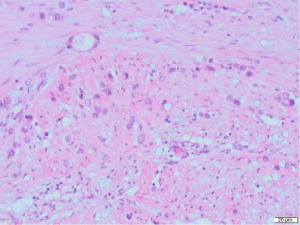
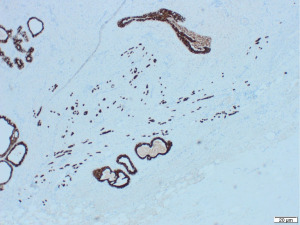
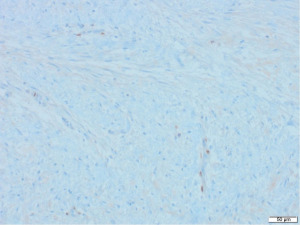
Following further discussion in the post-operative Breast MDT meeting, she then had a sentinel lymph node biopsy to assess for regional nodal status which was negative. The tumour’s final histological staging was reported as pT1b pN0. Regarding prognosis and risk of recurrence, it is difficult to make evidence-based predictions owing to the rarity of ILCs and PTs co-existing. However, the recurrence risk of non-metastatic low grade breast cancers (pT1a/b pN0, pM0), such as the ILC reported here, has been previously characterised, with overall survival and recurrence-free survival rates of 98.4% and 97.1% at 5-year follow up (11). Similarly, whilst previous studies have estimated the recurrence rate of borderline PTs to be 13% (12), the chance of recurrence in this specific instance is hard to predict owing to the rarity of PTs containing invasive carcinoma.
At 2 months following axillary surgery, the patient was well with fully healed wounds in the breast and axilla; no post-operative complications. She had had regular contact with the breast care nurses for support and advice. She was also referred to oncology for adjuvant radiotherapy to the breast and endocrine therapy. Six monthly ultrasonography surveillance and annual mammography for 2 years with follow-up in the breast clinic was also planned. To date, all surveillance imaging has shown no evidence of recurrent disease.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
PTs are a rare breast neoplasm comprising less than 1% of all breast tumours (1). They are classified as either ‘benign’, ‘borderline’ or ‘malignant’, with around 10% of cases being classified as malignant (2,3,5). PTs can be difficult to differentiate from fibroadenomas. Clinically, PTs present as a palpable lump and patients may report rapid growth (13). Ultrasound alone may not be sufficient to distinguish PT from fibroadenoma owing to the substantial overlap in sonographic characteristics (14). The presence clefts or round cysts within a solid mass is suggestive of a PT (15). Histological features typical of PT, rather than fibroadenoma, include: leaf-like architecture, increased stromal cellularity, stromal overgrowth, fragmentation and the presence of adipose tissue within the lesion (16). In this case, the crucial identification of radiological inconsistencies with the working diagnosis of fibroadenoma and an awareness of the possible confirmation bias conferred by the core-biopsy result enabled the experienced MDT to dictate the need for an excision biopsy to confirm a diagnosis of PT and ILC and therefore suggest further management (15).
The coexistence of PTs and invasive breast carcinoma or in situ carcinoma is rare, featuring in less than 1% of PTs (8,9). Where invasive carcinoma and PTs exist synchronously, ILC occurs less often than IDC (10). Only 5 other cases have been reported over the last 20 years to describe the presence of ILC within a PT (Table 1). Of this, only 2 feature ‘borderline’ type of PT and both of these contain invasive and in situ lobular carcinoma. Our case is the first to report the presence of exclusively ILC within a ‘borderline’ type PT.
Table 1
| Study | Age (years) | Duration of symptoms | Pathology | Surgical management |
|---|---|---|---|---|
| Chen et al. 2022 (17) | 43 | 3 years | ILC within benign PT | Nipple sparing mastectomy follow by total mastectomy with ANC |
| Shirah et al. 2011 (18) | 49 | Asymptomatic | ILC and LCIS within benign PT | Wide local excision followed by re-excision of margins and SLNB |
| Kodama et al. 2003 (19) | 47 | 10 years | ILC and LCIS within benign PT | Skin sparing mastectomy |
| Fischer et al. 2018 (20) | 40 | Unknown | ILC and LCIS within borderline PT | Lumpectomy with sentinel node biopsy followed by bilateral total mastectomy |
| Potdevin et al. 2016 (21) | 63 | Detected on routine clinical examination | ILC and LCIS within borderline PT | Excisional biopsy followed by Nipple sparing mastectomy |
| Current case | 40 | 2 months | ILC within borderline PT | Excisional biopsy followed by SLNB |
ILC, invasive lobular carcinoma; PT, phyllodes tumour; LCIS, lobular carcinoma in situ; ANC, axillary node clearance; SLNB, sentinel lymph node biopsy.
On comparing the clinical presentation with the 5 other reported cases, 2 were asymptomatic and discovered on routine examination or via breast screening and 3 were long-standing breast lumps (3, 10 and 10 years). For the latter 3 cases, it was suggested that the long growth period of the PT may have contributed to the development of the concomitant ILC (17-19). Our case conflicts this theory with only a 2-month history of a breast lump and an even shorter 1-week history of symptoms (discomfort and tightness). This perhaps suggests the possibility of simultaneous PT and ILC growth. Any theorised relationship between ILC and PT growth is ultimately speculative and a definitive link between the two conditions is not likely to be determined owing to the rarity of their coexistence.
The National Institute for Health and Care Excellence (NICE) guidelines dictate that patients aged over 30 with an unexplained breast lump, or patients over 50 with concerning unilateral nipple changes, are referred for specialist assessment within 2 weeks (22). This assessment comprises of the triple assessment (clinical examination, imaging and tissue biopsy) which informs diagnosis and management. In the case of PTs, no clear guidelines exist to define the optimal management and follow-up. Whilst mastectomy has historically been the treatment of choice, breast conserving surgery (BCS) with tumour free margins is now the generally accepted approach regardless of PT stage (23). In our case, excisional biopsy was performed as opposed to BCS or mastectomy as there was no definite pre-operative diagnosis of PT or invasive disease, and core biopsy had shown features in keeping with a fibroadenoma. As the tumour was fully excised with clear resection margins of a minimum of 4mm, no further excision of breast tissue was required. Second stage sentinel lymph node biopsy only was carried out to assess for nodal spread to the axilla.
Local recurrence of breast cancer is heavily influenced by the status of the resection margins (positive or negative). Despite this, systematic review by Lu et al. has shown that local recurrence is not significantly affected by margin status when the PT is ‘benign’ or ‘borderline’ (12). A resection margin of 1 cm is recommended by some studies, though others have suggested a margin of 1mm may be more appropriate, especially for ‘benign’ PT (12,24,25). The National Comprehensive Cancer Network recommends wide local excision with resection margins of 1 cm or greater for the surgical management of borderline/malignant PTs (26). This is considerably larger than the 1 mm minimum resection margin that is recommended by the National Institute for Health and Care Excellence for invasive or in situ breast cancer (27). The need for such a considerable resection margin of ≥h cm has been argued by some studies though a meta-analysis study by Thind et al. concluded that current available evidence suggests margins of less than 1 cm are adequate for the management of borderline or malignant PTs (28). This smaller resection margin also offers the benefit of better cosmetic outcomes especially in those with a smaller breast-to-tumour volume ratio.
Histological factors thought to predict local recurrence of PT include: stromal atypia, stromal mitoses, stromal overgrowth and stromal cellularity as well as tumour features such as tumour necrosis and tumour borders (12,29). Overall, systematic review and meta-analysis has suggested a local recurrence rate of 8% for benign PT, 13% for borderline PT and 18% for malignant PT (12). This highlights the importance of close follow up and surveillance regardless of the surgical management, tumour stage or resection margin (12).
Conclusions
This case raises awareness and acts as a reminder of the potential of invasive carcinoma to present in seemingly benign breast lumps. This case also emphasises the crucial role of the MDT and the attention paid to discordances in the triple assessment findings. It highlights why it is important not to succumb to confirmation biases when presented with a breast lump that has been shown to be a benign fibroadenoma on core biopsy despite radiological concerns for alternative diagnoses, such as a PT. Without the thorough and unbiased MDT discussion that took place, the decision to further investigate the seemingly benign breast lump in the form of a surgical excisional biopsy would have otherwise been overlooked, resulting in inadequate management and poorer outcomes. Due to the rarity of this case, the available literature on the topic is limited and there is scope for further research in this area. From the available literature, it seems that patient outcomes are better when PTs are ‘benign’ rather than ‘malignant’, irrespective of the presence of an invasive carcinoma or an in situ carcinoma (21). Ultimately, the niche nature of these coexisting diagnoses means that cases are, and must continue to be, managed by an experienced MDT on a case-by-case basis and seemingly benign breast lumps with discordant triple assessment findings must always undergo thorough MDT-led assessment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-52/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-52/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dyer NH, Bridger JE, Taylor RS. Cystosarcoma phylloides. Br J Surg 1966;53:450-5. [Crossref] [PubMed]
- Zhang Y, Kleer CG. Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch Pathol Lab Med 2016;140:665-71. [Crossref] [PubMed]
- Lakhani SR, Elis IO, Schnitt SJ, et al. World Health Organization Classification of Tumours of the Breast. 4th ed. Lakhani SR, Ellis IO, Schnitt SJ, et al. editors. World Health Organization Classification of tumours. Lyon: Internat. Agency for Research on Cancer, 2012;4:142-7.
- Parfitt JR, Armstrong C, O'malley F, et al. In-situ and invasive carcinoma within a phyllodes tumor associated with lymph node metastases. World J Surg Oncol 2004;2:46. [Crossref] [PubMed]
- Nio Y, Iguchi C, Tsuboi K, et al. Ductal carcinoma in situ arising within a benign phyllodes tumor: A case report with a review of the literature. Oncol Lett 2011;2:223-8. [Crossref] [PubMed]
- Panko N, Jebran AA, Gomberawalla A, et al. Invasive Ductal Carcinoma within a Benign Phyllodes Tumor. Am J Case Rep 2017;18:813-6. [Crossref] [PubMed]
- Bernstein L, Deapen D, Ross RK. The descriptive epidemiology of malignant cystosarcoma phyllodes tumors of the breast. Cancer 1993;71:3020-4. [Crossref] [PubMed]
- Co M, Tse GM, Chen C, et al. Coexistence of Ductal Carcinoma Within Mammary Phyllodes Tumor: A Review of 557 Cases From a 20-year Region-wide Database in Hong Kong and Southern China. Clin Breast Cancer 2018;18:e421-5. [Crossref] [PubMed]
- Tan PH, Jayabaskar T, Chuah KL, et al. Phyllodes tumors of the breast: the role of pathologic parameters. Am J Clin Pathol 2005;123:529-40. [Crossref] [PubMed]
- To H, Ong BS, Dodd T, et al. Synchronous malignant phyllodes tumour and invasive lobular carcinoma-case report and review. J Surg Case Rep 2018;2018:rjy258. [Crossref] [PubMed]
- Dalenc F, Penault-Llorca F, Cohen M, et al. Daily Practice Management of pT1a-b pN0 Breast Carcinoma: A Prospective French ODISSEE Cohort Study. Clin Breast Cancer 2017;17:107-16. [Crossref] [PubMed]
- Lu Y, Chen Y, Zhu L, et al. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann Surg Oncol 2019;26:1263-75. [Crossref] [PubMed]
- Jacklin RK, Ridgway PF, Ziprin P, et al. Optimising preoperative diagnosis in phyllodes tumour of the breast. J Clin Pathol 2006;59:454-9. [Crossref] [PubMed]
- Chao TC, Lo YF, Chen SC, et al. Sonographic features of phyllodes tumors of the breast. Ultrasound Obstet Gynecol 2002;20:64-71. [Crossref] [PubMed]
- Wiratkapun C, Piyapan P, Lertsithichai P, et al. Fibroadenoma versus phyllodes tumor: distinguishing factors in patients diagnosed with fibroepithelial lesions after a core needle biopsy. Diagn Interv Radiol 2014;20:27-33. [Crossref] [PubMed]
- Lee AH, Hodi Z, Ellis IO, et al. Histological features useful in the distinction of phyllodes tumour and fibroadenoma on needle core biopsy of the breast. Histopathology 2007;51:336-44. [Crossref] [PubMed]
- Chen YH, Tu YL, Chen HK, et al. Invasive lobular carcinoma enclosed by a benign phyllodes tumor: A case report. Int J Surg Case Rep 2022;91:106804. [Crossref] [PubMed]
- Shirah GR, Lau SK, Jayaram L, et al. Invasive lobular carcinoma and lobular carcinoma in situ in a phyllodes tumor. Breast J 2011;17:307-9. [Crossref] [PubMed]
- Kodama T, Kameyama K, Mukai M, et al. Invasive lobular carcinoma arising in phyllodes tumor of the breast. Virchows Arch 2003;442:614-6. [Crossref] [PubMed]
- Fischer KM. S J Brooks J, Ugras SK. Invasive lobular carcinoma involving a borderline phyllodes tumor. Breast J 2018;24:1076-7. [Crossref] [PubMed]
- Potdevin LB, Alsaati G, Sidawy M, et al. Management of synchronous phyllodes tumors with invasive breast carcinoma: A review of the literature and a case report breast. Cancer Treat Res Commun 2016;9:134-8. [Crossref]
- National Institute for Health and Care Excellence (NICE). Scenario: Referral for breast cancer | Management | Breast cancer - recognition and referral | CKS | NICE. 2020 [cited 2021 Nov 12]. Available online: https://cks.nice.org.uk/topics/breast-cancer-recognition-referral/management/referral-for-breast-cancer/
- Mitus JW, Blecharz P, Jakubowicz J, et al. Phyllodes tumors of the breast. The treatment results for 340 patients from a single cancer centre. Breast 2019;43:85-90. [Crossref] [PubMed]
- Tremblay-LeMay R, Hogue JC, Provencher L, et al. How Wide Should Margins Be for Phyllodes Tumors of the Breast? Breast J 2017;23:315-22. [Crossref] [PubMed]
- Lim RS, Cordeiro E, Lau J, et al. Phyllodes Tumors-The Predictors and Detection of Recurrence. Can Assoc Radiol J 2021;72:251-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Breast Cancer. Clinical Practice Guidelines in Oncology (NCCN Guidelines). Version 5.2020. Plymouth, PA; 2020 [cited 2022 Dec 15]. Available online: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf
- National Institute for Health and Care Excellence (NICE). Recommendations | Early and locally advanced breast cancer: diagnosis and management | Guidance | NICE. NICE; 2018 [cited 2021 Nov 13]. Available online: https://www.nice.org.uk/guidance/ng101/chapter/Recommendations#followup
- Thind A, Patel B, Thind K, et al. Surgical margins for borderline and malignant phyllodes tumours. Ann R Coll Surg Engl 2020;102:165-73. [Crossref] [PubMed]
- Tan PH, Thike AA, Tan WJ, et al. Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 2012;65:69-76. [Crossref] [PubMed]
Cite this article as: Hudson-Phillips S, Cox K, Williams L, Noor L. Incidental finding of invasive lobular carcinoma within a borderline phyllodes tumour of the breast: a case report. AME Med J 2023;8:10.