
Ventricular fibrillation during pulmonary artery catheter placement in a patient with Stanford type A aortic dissection: a case report
Highlight box
Key findings
• We encountered a case of ventricular fibrillation (VF) during pulmonary artery catheter (PAC) placement in a patient with a Stanford type A aortic dissection. The patient was treated appropriately. However, it is unclear whether PAC insertion was appropriate for this case.
What is known and what is new?
• The indications for pulmonary artery catheterization are controversial and the routine use of a PAC is not recommended because of the complications associated with this invasive procedure.
• The placement of a PAC in patients with an enlarged ascending aorta is very difficult. VF is more likely to occur in cases of suspected myocardial ischemia.
What is the implication, and what should change now?
• The indications for pulmonary artery catheterization in patients with an enlarged ascending aorta and suspected myocardial ischemia should be carefully considered. Furthermore, the use of PACs should be avoided in these cases.
Introduction
Background
The continuous monitoring of hemodynamics is crucial for maintaining adequate tissue perfusion and oxygen levels in patients with severe cardiac disease. A pulmonary artery catheter (PAC) can continuously measure hemodynamic variables such as pulmonary artery pressure, pulmonary artery wedge pressure, central venous pressure, cardiac output, and mixed venous oxygen saturation (SvO2). Therefore, the PAC is an effective monitor in cardiac patients. However, the use of PAC can possibly cause severe to fatal complications as previously reported (1). Similarly, we experienced fatal arrythmia during PAC insertion. Therefore, its routine use in cardiac surgery remains controversial due to the complications associated with the invasive procedure.
Rationale and knowledge gap
Surveys have reported that about one-third of respondents routinely use PACs in all cases of cardiopulmonary bypass (CPB) surgery (2). In fact, in our hospital, we routinely use PACs in all cardiac surgery cases. Presently, there are no comprehensive guidelines outlining the indication criteria for the use of PAC in cardiac surgery. The use of PAC should be indicated based on patient risk, surgical procedure, and clinical circumstances.
The supervision of a specialist and use of an ultrasound guide is recommended and necessary for the safe insertion of the central venous line and placement of PAC (3).
Objective
We report an incidence of ventricular fibrillation (VF) occurring during catheter insertion, despite the utilization of an ultrasound guide, in a patient with Stanford type A acute aortic dissection. We discuss the risks of routine PAC use in patients with normal left ventricular function, an enlarged ascending aorta, and suspected myocardial ischemia. The case was prepared according to the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-89/rc).
Case presentation
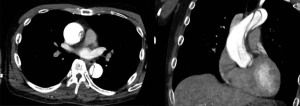
A 48-year-old man (height, 171 cm; weight, 75 kg) with severe untreated hypertension was admitted for emergency complaints of chest pain and ST-segment elevation from V1 to V4 on electrocardiogram, and was suspected for acute myocardial infarction on December 1st, 2020. Angiography revealed a Stanford type A acute aortic dissection with entry into the ascending aorta, and the patient underwent emergency surgery on the same day. Preoperative transthoracic echocardiography showed good left ventricular wall motion without any valvular disorder. The dissected lumen extended to the aortic root. Contrast-enhanced computed tomography (CT) revealed that the aortic root was enlarged to 45 mm (Figure 1). Hematological examination revealed no abnormalities.
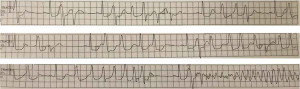
General anesthesia was induced and maintained with propofol, sevoflurane fentanyl, and rocuronium. Tracheal intubation was performed after muscle reluctance was achieved. Transesophageal echocardiography (TEE) was performed after tracheal intubation. Regional oxygen saturation (rSO2) and bispectral index (BIS) monitoring was performed on both sides of the forehead. An arterial catheter was placed in both radial arteries to measure blood pressure. A central venous catheter and a PAC were placed in the right internal jugular vein. We attempted to insert the PAC under TEE guidance, but it was difficult to reach the right ventricle. After the catheter was repeatedly withdrawn and inserted, it reached the right atrium at the fourth insertion. However, premature ventricular contractions (PVCs) occurred, thus resulting in hypotension, which then shifted to VF (Figure 2). We immediately initiated chest compressions and electrical defibrillation (biphasic 150 J) and administered 100 mg of lidocaine and 20 mg of nifekalant. Sinus rhythm returned after the fourth electrical defibrillation. Electrolyte abnormalities were ruled out on the basis of the results of blood gas tests. The duration of PAC placement time was 7 min, and the duration of VF was 2 min 30 s. We stopped advancing the PAC into the pulmonary artery and initiated the surgery. Intraoperative TEE revealed that the dissected lumen extended to the aortic root. The intimal flap covered the origin of the left coronary artery, thus resulting in reduced coronary perfusion (Figure 3).
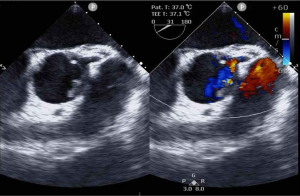
Ascending aortic arch replacement was successfully completed, and the PAC was placed in the pulmonary artery under TEE guidance after CPB. The CPB time was 216 min, operation time was 453 min, and anesthesia time was 488 min. Intraoperative BIS monitoring and rSO2 measurement showed normal values.
The patient was transferred to the intensive care unit (ICU) postoperatively. Nicardipine was continuously administered as an intravenous infusion of 1 to 3 mg/h to maintain systolic blood pressure of <110 mmHg until discharge from the ICU. Amiodarone (600 mg/day) and landiolol (1–3 µg/kg/min) were administered to prevent VF recurrence until postoperative day (POD) 1. The patient was extubated on POD 1 and discharged from the ICU on POD 3. No fatal arrhythmias occurred during the study period. The patient was discharged without any neurological symptoms or other complications on POD 15.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
Various complications associated with PAC placement have been reported including infection, adjacent aortic injury, pulmonary artery injury, pulmonary emboli, heart valve injury, and arrhythmia (4). Arrhythmias related to PAC originate from ectopic atrial and ventricular beats, atrial and ventricular tachycardias, and conduction abnormalities. The incidence of arrhythmias during PAC placement has been reported to range from 12.5% to 70%. PVCs are the most common arrhythmias (52–68%), followed by non-sustained ventricular tachycardias. Even though ventricular tachycardia and fibrillation are rare (occurring in less than 1% of cases), they are always fatal once they occur (5,6). In cases involving cardiogenic shock and severe cases, PAC does not confer significant benefits with respect to reduced mortality, shorter ICU stays and hospitalization times, and lower medical costs in the ICU (7,8). A meta-analysis of 13 randomized clinical trials found that the insertion of a PAC in critically ill patients had no benefits or disadvantages (9). Meanwhile, various complications are associated with PAC insertion, and the benefits of this procedure are unclear. Therefore, the indications for PAC insertion remain controversial.
In our case, PAC insertion was difficult to advance the catheter into the right ventricle and PAC placement time was increased. During withdrawal and insertion of the catheter, VF occurred. In the case of Stanford type A aortic dissection with coronary artery blood flow restriction due to arterial intimal flap obstruction, VF occurred following an arrhythmia due to PAC insertion. In cases with normal left ventricular function, PAC should be avoided because the benefits of PAC are unclear.
Strengths and limitations
This case report’s strength is that it describes a rare but fatal complication of PAC insertion in an aortic dissection case. We suggested a reconsideration of the routine use of PAC and administered antiarrhythmics, vasodilators, and beta-blocker to maintain good hemodynamic status without VF recurrence in the ICU. However, this case report also has a limitation. The other causes of VF should be acknowledged including low coronary perfusion pressure, arrhythmia, and hypotension on top of other contributing factors
Comparison with similar researches
PAC can continuously measure hemodynamic parameters. These parameters can clarify the reasons for the intervention and its effectiveness. Some studies have suggested that a PAC is useful for postoperative management of cardiac surgery in the ICU (10).
The reported complications of PACs can be categorized into four groups: central venous access, complications related to catheter procedures, complications associated with the long-term presence of the catheter, and errors resulting from incorrect interpretation of PAC-derived data. Sangkum et al. summarized various complications in the manuscript (11). For central venous puncture, Seldinger’s technique using a guidewire with ultrasound is safer and less risky for unintentional punctures (4). The advancement of a PAC under TEE guidance is also safer than conventional methods of checking the pressure waveform (12); this is why TEE insertion is preferable before PAC insertion. Catheterization may occasionally cause a right bundle branch block. Patients with a preoperative left bundle branch block require more attention because they may develop a complete atrioventricular block, thus necessitating pacemaker treatment (13).
In our case, VF was treated with immediate chest compressions, electrical cardioversion, and antiarrhythmics. Lidocaine, nifekalant, and amiodarone are the typical antiarrhythmic agents used for VF. Lidocaine is associated with lower survival during hospitalization than amiodarone, but the two agents show no significant difference in relation to survival and discharge (14). Nifekalant is reportedly more effective for defibrillation than lidocaine. Nifekalant is a pure delayed rectifier potassium channel blocker that prolongs the refractory period. Its major advantage is that it has little effect on sodium or calcium channels; therefore, it has no negative inotropic or vasodilating effects (15). Nifekalant and amiodarone improved 24-h survival to a similar degree in a multicenter cohort study. The time from drug administration to successful defibrillation was significantly shorter with nifekalant than with amiodarone (16). The effects of nifekalant and amiodarone in a porcine model of 4-min cardiac arrest due to VF were investigated in one study, which showed that the return of spontaneous circulation (ROSC) and 24 h survival rates were not different between the two groups, but the coronary perfusion pressure at 30 min after ROSC was significantly higher with nifekalant than with amiodarone. The difference in coronary perfusion pressure immediately after resuscitation may have affected the time to ROSC (17). In our case, nifekalant administration contributed to the return to sinus rhythm.
In intensive care management, the control of blood pressure and the prevention of arrhythmia recurrence are important for maintaining organ blood perfusion in patients after aortic dissection surgery and VF resuscitation. Our patient received continuous amiodarone, landiolol, and electrolyte management to prevent VF recurrence. Amiodarone administration is considered useful for survival according to the 2015 American Heart Association guidelines (14). However, it failed to achieve better survival to discharge and neurological outcomes compared with placebo in a randomized controlled trial in 2016 (18). Serum potassium levels during the first 72 h of treatment and the use of beta-blocking agents were significantly associated with survival from out-of-hospital cardiac arrest (19). Beta-blocker administration during cardiac surgery reduces the incidence of atrial fibrillation and ventricular arrhythmias (20). In our case, the patient maintained good hemodynamic status without VF recurrence owing to the administration of amiodarone and beta-blockers in the ICU.
Explanations of findings
In our case, PAC insertion was attempted under TEE guidance, but it was difficult to advance the catheter into the right ventricle probably because of the right atrial compression of the enlarged aortic root. VF consequently occurred during the repeated withdrawal and insertion of the catheter. Electrocardiography revealed that the ST segment was elevated in V1–V4, and coronary artery occlusion due to the arterial intimal flap may have been restricted to the coronary artery blood flow. VF may occur because of the hypotension induced by anesthetic induction and by the mechanical stimulation of the myocardium by the PAC, which results in extrasystole and exacerbation of myocardial ischemia. The enlargement of the proximal ascending aorta may compress the right atrium and right ventricle. A longer PAC insertion time leads to a higher incidence of ventricular arrhythmia (21).
Implications and actions needed
In our case, the enlargement of the proximal ascending aorta can provide more challenge to PAC insertion. Longer insertion time leads to a higher incidence of ventricular arrhythmia (21). The arterial intimal flap in the aortic root can obstruct the coronary artery. Mechanical stimulation to the myocardium by the PAC can cause extrasystole, hypotension, and exacerbation of myocardial ischemia, resulting in VF. In an enlarged ascending aorta with normal left ventricular function, PAC should be avoided to prevent fatal arrythmias. Even if PAC is used, it is best to stop in the right atrium until surgical repair and advance it into the pulmonary artery only after the surgical repair.
Conclusions
In conclusion, we observed VF during PAC placement in a patient with a Stanford type A aortic dissection. VF was treated appropriately, and the surgery was successfully completed. Postoperative antiarrhythmic care ensured a stable hemodynamic status. We should not use a PAC in patients with an enlarged ascending aorta and suspected myocardial ischemia.
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-89/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-89/coif). IK serves as an unpaid editorial board member of AME Medical Journal from February 2023 to January 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Benito-Saz P, Garrido A, Quintana-Villamandos B, et al. Perforation of the left ventricle wall due to the insertion of a pulmonary artery catheter. A case report. Rev Esp Anestesiol Reanim 2019;66:528-32. (Engl Ed). [Crossref] [PubMed]
- Judge O, Ji F, Fleming N, et al. Current use of the pulmonary artery catheter in cardiac surgery: a survey study. J Cardiothorac Vasc Anesth 2015;29:69-75. [Crossref] [PubMed]
- Alsatli RA. Ultrasound-guided insertion of pulmonary artery catheter: Case report. J Saudi Heart Assoc 2010;22:223-4. [Crossref] [PubMed]
- Practice guidelines for pulmonary artery catheterization: an updated report by the American Society of Anesthesiologists Task Force on Pulmonary Artery Catheterization. Anesthesiology 2003;99:988-1014. [Crossref] [PubMed]
- Evans DC, Doraiswamy VA, Prosciak MP, et al. Complications associated with pulmonary artery catheters: a comprehensive clinical review. Scand J Surg 2009;98:199-208. [Crossref] [PubMed]
- Shah KB, Rao TL, Laughlin S, et al. A review of pulmonary artery catheterization in 6,245 patients. Anesthesiology 1984;61:271-5. [Crossref] [PubMed]
- Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a andomized controlled trial. Lancet 2005;366:472-7. [Crossref] [PubMed]
- Rajaram SS, Desai NK, Kalra A, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2013;2013:CD003408. [Crossref] [PubMed]
- Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005;294:1664-70. [Crossref] [PubMed]
- Szabo C, Betances-Fernandez M, Navas-Blanco JR, et al. PRO: The pulmonary artery catheter has a paramount role in current clinical practice. Ann Card Anaesth 2021;24:4-7. [Crossref] [PubMed]
- Sangkum L, Liu GL, Yu L, et al. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth 2016;30:461-80. [Crossref] [PubMed]
- Essandoh M. Transesophageal Echocardiography Should Be Considered During Pulmonary Artery Catheter Insertion in Cardiac Surgery. J Cardiothorac Vasc Anesth 2017;31:e93. [Crossref] [PubMed]
- Morris D, Mulvihill D, Lew WY. Risk of developing complete heart block during bedside pulmonary artery catheterization in patients with left bundle-branch block. Arch Intern Med 1987;147:2005-10. [Crossref] [PubMed]
- Dorian P, Cass D, Schwartz B, et al. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med 2002;346:884-90. [Crossref] [PubMed]
- Katoh T, Mitamura H, Matsuda N, et al. Emergency treatment with nifekalant, a novel class III anti-arrhythmic agent, for life-threatening refractory ventricular tachyarrhythmias: post-marketing special investigation. Circ J 2005;69:1237-43. [Crossref] [PubMed]
- Amino M, Inokuchi S, Nagao K, et al. Nifekalant Hydrochloride and Amiodarone Hydrochloride Result in Similar Improvements for 24-Hour Survival in Cardiopulmonary Arrest Patients: The SOS-KANTO 2012 Study. J Cardiovasc Pharmacol 2015;66:600-9. [Crossref] [PubMed]
- Ji XF, Li CS, Wang S, et al. Comparison of the efficacy of nifekalant and amiodarone in a porcine model of cardiac arrest. Resuscitation 2010;81:1031-6. [Crossref] [PubMed]
- Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med 2016;374:1711-22. [Crossref] [PubMed]
- Skrifvars MB, Pettilä V, Rosenberg PH, et al. A multiple logistic regression analysis of in-hospital factors related to survival at six months in patients resuscitated from out-of-hospital ventricular fibrillation. Resuscitation 2003;59:319-28. [Crossref] [PubMed]
- Blessberger H, Lewis SR, Pritchard MW, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity in adults undergoing cardiac surgery. Cochrane Database Syst Rev 2019;9:CD013435. [PubMed]
- Satoh H, Miyata Y, Hayasaka T, et al. An analysis of the factors producing multiple ventricular arrhythmias during pulmonary artery catheterization. Ann Card Anaesth 2017;20:141-4. [Crossref] [PubMed]
Cite this article as: Kochiyama T, Kusano Y, Yamaguchi A, Sakuraya S, Kawagoe I. Ventricular fibrillation during pulmonary artery catheter placement in a patient with Stanford type A aortic dissection: a case report. AME Med J 2023;8:8.

