
Surgical management of placenta percreta complicated by bladder invasion: a case report
Highlight box
Key findings
• This case demonstrates placental invasion of the urinary bladder treated via cesarean section, hysterectomy, and invasive placental tissue dissection.
• Postoperative course was complicated by necrosis of vaginal cuff and cystotomy repair, resulting in a large vesicovaginal fistula (VVF).
• Vaginal cuff and bladder were closed in a manner that avoided overlapping suture lines.
• A flap of omentum was interposed between vagina and bladder to decrease risk of VVF reformation.
What is known and what is new?
• Urologic complications of PAS are relatively rare but potentially severe.
• This is the first case to result in the combination of massive hemorrhage, cystotomy, bladder necrosis, vaginal cuff necrosis, and VVF formation.
What is the implication, and what should change now?
• Multidisciplinary preoperative consultation is crucial to prepare for life-threatening complications of PAS.
• Use of pre-vesicouterine dissection bladder filling, hemostatic clips during dissection, and “Triple P Procedure” can preserve bladder tissue and function.
Introduction
Placenta accreta spectrum (PAS) is characterized by abnormal placental invasion due to insufficient or complete loss of intervening decidua. Subtypes of PAS are classified by the depth of invasion: placenta creta invades through the endometrium and attaches to the myometrium, placenta increta invades into the myometrium, and placenta percreta invades through the uterine wall, often invading into nearby structures, most commonly the bladder (1-3). Urologic complications of PAS, including inadvertent cystotomy during hysterectomy, massive hemorrhage requiring therapeutic embolization and subsequent bladder necrosis, vaginal cuff necrosis, and vesicovaginal fistula formation, have all been described as individually rare in case reports (4-7), but are particularly notable in combination. To our knowledge, this is the first case report documenting the simultaneous combination of such complications (see timeline visualized in Figure 1). We present this case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-107/rc).
Case presentation
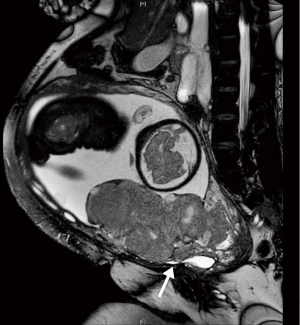
A 30-year-old woman with a history of three prior cesarian sections presented at 7 weeks gestation with a possible ectopic pregnancy with implantation into the cesarean scar. She was lost to follow up until she returned at 29 weeks gestation, at which time ultrasound demonstrated placenta percreta with possible extension to the bladder. MRI confirmed placenta previa with full thickness placental invasion of the anterior lower uterine segment. However, bladder wall invasion was not clearly visualized (Figure 2).
Our institution’s interdisciplinary placenta percreta team, including members from high-risk obstetrics, anesthesiology, urology, interventional radiology, and the blood bank team, planned cesarean section and hysterectomy at 34 weeks 4 days gestation. However, the patient arrived in the emergency room one week early with painful contractions every two minutes for two hours. She was therefore taken to the operating room. After induction of spinal anesthesia and placement of a central line, cystoscopy was performed, and ureteral catheters were placed. There was no gross placental invasion noted in the bladder.
After a cesarian section was performed and the baby was delivered, the subsequent hysterectomy was complicated by class 4 obstetric hemorrhage, with an estimated blood loss of 40 L, requiring activation of a massive transfusion protocol. The patient received 76 units of packed red blood cells, 36 units of fresh frozen plasma, 18 units of cryoprecipitate and 14 units of platelets. She suffered from multiple periods of severe hypotension during the operation. Diffuse brisk bleeding required removal of adherent placental tissues embedded into adjacent structures including the pelvic side wall and the bladder. Once the uterus was removed, a 10 cm inadvertent cystotomy was noted at the bladder dome, without injury to trigone or ureters. The bladder edges were approximated in two running layers of absorbable suture. The bladder closure was tested with 150 cc of normal saline, which confirmed a water-tight closure. Due to concern for ongoing bleeding, the patient was taken from the operating room directly to the interventional radiology suite, where she underwent bilateral internal iliac (hypogastric) artery embolization with Gelfoam. Right angioembolization was performed proximally on the artery in the right side and distally on the left.
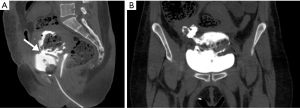
She was transferred to the intensive care unit where she stabilized. She was extubated on post-operative day (POD) #1 and was ultimately discharged home on POD #8 with a foley catheter, acetaminophen 650 mg every 6 hours (q6h) for 10 days, diclofenac gel 1% q8h for 21 days, nitrofurantoin 200 mg q12h for 6 days, oxycodone 5 mg q4h prn for 6 days, tamsulosin 0.4 mg q24h for 3 days, and oxybutynin 15 mg q24h for 3 days. On POD #12, a fluoroscopic cystogram revealed small volume intraperitoneal contrast extravasation at the right dome of the bladder with possible vesicovaginal fistula. The catheter was left in place. On POD #17, the patient presented to the Emergency Room with burning pain at the foley site. A CT cystogram showed a 4 cm by 1 cm defect involving the midline bladder dome, compatible with intraperitoneal bladder leak (Figure 3). The extravasated contrast was seen to communicate with the vaginal cuff. She had no pelvic fluid collections or abscess. Due to her recent major surgery, she was managed conservatively at this time with continued foley catheter drainage as reconstructive surgery was planned. On POD #35, a repeat CT cystogram showed continued large volume extravasation, and post-drainage film showed clearance of contrast through the foley catheter.
On POD #41, she was brought back to the operating room. An exploratory laparotomy revealed complete dehiscence of the cystotomy repair with a walled-off urinoma in communication with a completely dehisced necrotic vaginal cuff, forming a large vesicovaginal fistula. The vaginal cuff was mobilized away from the bladder, debrided, and closed in a figure-of-eight fashion. A vaginal exam revealed at least 8 cm of vaginal length. The bladder edges appeared necrotic, and so a partial cystectomy was performed to excise the devitalized portion of the bladder. The bladder was closed vertically to avoid overlapping suture lines with the vagina. A running suture was used for the inner mucosal layer followed by interrupted figure-of-eight sutures for the muscularis layer. The bladder closure was tested with 150 cc of normal saline, and there was no leak. A flap of peritoneum from the reflection over the bladder was mobilized and interposed over the suture line. A Jackson-Pratt drain and foley catheter were placed. The patient recovered well and was discharged home 2 days after surgery with acetaminophen 975 mg q6h prn for 30 days, ketorolac 10 mg q6h for 20 days, oxybutynin 15 mg q24h for 30 days.
Two weeks post-operatively, a cystogram revealed no leak and the foley catheter was removed. At one month post-operatively, cystoscopy revealed an intact suture line and capacity of at least 200 cc. At her most recent follow-up, approximately 3 months after the repair, she had no urinary complaints and reported feeling like herself again.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Discussion
We present a particularly devastating case of a 30-year-old woman who suffered severe morbidity related to PAS with invasion of the urinary bladder. This case demonstrated massive hemorrhage requiring bilateral hypogastric angioembolization, later complicated by bladder dome and vaginal cuff necrosis, urinoma and vesicovaginal fistula (VVF).
This case report details urological complications of PAS that are rare when experienced in isolation, but their co-occurrence makes them particularly remarkable. As such, the case illuminates several important considerations for patients with PAS, including the relevant medical history diagnostic testing, the importance of an interdisciplinary team approach, and the need for preparation for potentially devastating surgical complications. This report is limited by the lack of information around the patient’s loss to follow-up as well as lack of long-term outcomes on her the quality of life.
The most common urologic injury in PAS surgery is cystotomy. Interestingly, the International Federation of Gynecology and Obstetrics (FIGO) guidelines recommend intentional cystotomy in placenta percreta with invasion of the urinary bladder because it exposes the extent of the placental invasion, defines dissection planes, and determination of whether resection at the posterior bladder wall is needed (2,8-11).
Maximal bladder preservation is important in the surgical management of PAS with bladder invasion. Prior to dissection of the vesicouterine plane, filling the bladder with saline allows better visualization of the border between the bladder and uterus. During the dissection, hemostatic clips and absorbable sutures can be employed to minimize bleeding, which is most often caused by aberrant placental vessels located in the superficial portion of the vesicouterine pouch (8).
In a technique called the “Triple P Procedure”, if there is posterior bladder wall involvement, the invading placental tissue is left in-situ, and confirmation of placental tissue absorption is confirmed at 8 weeks (12). This avoids operative trauma to the bladder and has been associated with a lower rate of postpartum hemorrhage and hysterectomy, although blood loss was similar to standard techniques. A previous study has also suggested utilizing adjuvant methotrexate post-partum when the placental tissue is left in-situ to increase the rate of placental absorption by decreasing trophoblast activity and placental vascularity (13).
Bladder necrosis after PAS and cystotomy repair is particularly rare, with only a few cases ever reported. Surgical dogma around embolization for postpartum hemorrhage asserts that necrosis should not be expected after embolization due to the vast collaterals arising from branches of the IIA and other vessels in the pelvic region (14). In fact, proximal embolization of the IIA may fail to control PAS-associated postpartum hemorrhage because of these numerous collateral vessels (15). Despite this, some postulate that bladder necrosis can occur if the uterine arteries give rise to the inferior vesical arteries (4).
Rates of vaginal cuff necrosis and VVF are exceedingly low. One study found that only 2 out of 66 (3.0%) patients with placenta percreta who underwent hysterectomy developed a VVF (6). Our patient’s fistula resulted from simultaneous cystotomy necrosis and vaginal cuff dehiscence. VVFs have also been noted in patients with significant pelvic blood loss due to regional trauma or vascular injury (2,7,16,17).
The principles of optimal surgical repair for VVF include favorable tissue conditions free from infection and inflammation, complete excision of the fistula tract, a tension-free anastomosis, multi-layered closure that avoids overlapping suture lines and the interposition of vascularized tissue (18). Several techniques exist for managing VVF, including using various flaps and grafts such as omentum, peritoneum, labial fat pad or gracilis muscle. The omental flap is particularly versatile as it can be harvested both abdominally or vaginally and can cover large defects (19). The lack of randomized controlled trials in the literature presents a challenge to understanding whether bladder necrosis is a common complication of IIA embolization in placenta percreta (5,20).
Conclusions
PAS with invasion into the urinary bladder can result in severe urologic complications. To our knowledge, we present the first case of a particularly morbid case of PAS with resulting urologic complications including cystotomy, bladder necrosis and vesicovaginal fistula formation. There are several surgical techniques that can be employed to preserve bladder tissue and function, including filling the bladder prior to vesicouterine dissection, the use of hemostatic clips during dissection, and the “Triple P Procedure”. In the weeks post-operation, providers should hold a high index of suspicion for bladder necrosis and fistula formation, regardless of its low incidence. Future research should investigate approaches that optimize patient-specific vascular anatomy and early detection of necrosis and fistula formation would greatly benefit patients with placenta percreta.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-107/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-107/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-107/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this case report and accompanying images was not obtained from the patient or the relatives after all possible attempts were made.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abotorabi S, Chamanara S, Oveisi S, et al. Effects of Placenta Location in Pregnancy Outcomes of Placenta Accreta Spectrum (PAS): A Retrospective Cohort Study. J Family Reprod Health 2021;15:229-35. [Crossref] [PubMed]
- Konijeti R, Rajfer J, Askari A. Placenta percreta and the urologist. Rev Urol 2009;11:173-6. [PubMed]
- Placenta Accreta Spectrum: The American College of Obstetricians and Gynecologists; 2021. Available online: https://www.acog.org/en/clinical/clinical-guidance/obstetric-care-consensus/articles/2018/12/placenta-accreta-spectrum
- Wu WJ, Smith AD, Okeke Z. Bladder Necrosis Associated with Placenta Accreta, Embolization, and Repair of Cystotomies. J Endourol Case Rep 2015;1:24-6. [Crossref] [PubMed]
- Bishop S, Butler K, Monaghan S, et al. Multiple complications following the use of prophylactic internal iliac artery balloon catheterisation in a patient with placenta percreta. Int J Obstet Anesth 2011;20:70-3. [Crossref] [PubMed]
- Clausen C, Lönn L, Langhoff-Roos J. Management of placenta percreta: a review of published cases. Acta Obstet Gynecol Scand 2014;93:138-43. [Crossref] [PubMed]
- Washington S, Osterberg EC, Elliott SP, et al. Acute Bladder Necrosis after Pelvic Arterial Embolization for Pelvic Trauma: Lessons Learned from Two Cases of Immediate Postembolization Bladder Necrosis. Case Rep Urol 2016;2016:7594192. [Crossref] [PubMed]
- Allen L, Jauniaux E, Hobson S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. Int J Gynaecol Obstet 2018;140:281-90. [Crossref] [PubMed]
- Erfani H, Salmanian B, Fox KA, et al. Urologic morbidity associated with placenta accreta spectrum surgeries: single-center experience with a multidisciplinary team. Am J Obstet Gynecol 2022;226:245.e1-5. [Crossref] [PubMed]
- Ibrahim MA, Liu A, Dalpiaz A, et al. Urological Manifestations of Placenta Percreta. Curr Urol 2015;8:57-65. [Crossref] [PubMed]
- Jain N, Patwardhan S, Jain HM, et al. Surgical strategies for placenta percreta invading the bladder and review of literature. Afr J Urol 2020;26: [Crossref]
- Teixidor Viñas M, Belli AM, Arulkumaran S, et al. Prevention of postpartum hemorrhage and hysterectomy in patients with morbidly adherent placenta: a cohort study comparing outcomes before and after introduction of the Triple-P procedure. Ultrasound Obstet Gynecol 2015;46:350-5. [Crossref] [PubMed]
- Zhang C, Li H, Zuo C, et al. Retrospective analysis: Conservative treatment of placenta increta with methotrexate. J Obstet Gynaecol Res 2018;44:907-13. [Crossref] [PubMed]
- Shrestha R, Shrestha S, Sitaula S, et al. Anatomy of Internal Iliac Artery and Its Ligation to Control Pelvic Hemorrhage. JNMA J Nepal Med Assoc 2020;58:826-30. [Crossref] [PubMed]
- Chou YJ, Cheng YF, Shen CC, et al. Failure of uterine arterial embolization: placenta accreta with profuse postpartum hemorrhage. Acta Obstet Gynecol Scand 2004;83:688-90. [Crossref] [PubMed]
- Anderson DJ, Liu H, Kumar D, et al. Placenta Percreta Complications. Cureus 2021;13:e18842. [PubMed]
- Marín-Sánchez P, Sánchez-Ferrer ML, Machado-Linde F. Conservative management of vesico-vaginal fistula after uterine and partial bladder necrosis due to embolization as a treatment for postpartum hemorrhage. Int Urogynecol J 2015;26:773-4. [Crossref] [PubMed]
- Hemal AK, Kolla SB, Wadhwa P. Robotic reconstruction for recurrent supratrigonal vesicovaginal fistulas. J Urol 2008;180:981-5. [Crossref] [PubMed]
- Stamatakos M, Sargedi C, Stasinou T, et al. Vesicovaginal fistula: diagnosis and management. Indian J Surg 2014;76:131-6. [Crossref] [PubMed]
- Peng Y, Jiang L, Peng C, et al. The application of prophylactic balloon occlusion of the internal iliac artery for the treatment of placenta accreta spectrum with placenta previa: a retrospective case-control study. BMC Pregnancy Childbirth 2020;20:349. [Crossref] [PubMed]
Cite this article as: Green BW, Zhu M, Loloi J, Patel RD, Small AC. Surgical management of placenta percreta complicated by bladder invasion: a case report. AME Med J 2023;8:19.

