
Alcohol-based hand sanitizer’s effect on hand barrier function induced Staphylococcus lugdunensis aortic and mitral valve endocarditis: a case report
Highlight box
Key findings
• Excessive application of ABHS without moisturizer can impair skin barrier integrity and function.
• S. Lugdunensis bacteremia causes aggressive and destructive left sided native valve endocarditis associated with high mortality.
What is known and what is new?
• ABHS has ability to denature and coagulate proteins, which induce lysis of the viral particle. This reduces the infectivity of the virus.
• Excessive use of ABHS can result in dry hands and skin damage, causing irritant or allergic contact dermatitis. This will increase the risk of local or systemic infection, which contradicts the original purpose of ABHS.
What is the implication and what should change now?
• In a pandemic, hand hygiene and masking are public health infection control measures to prevent the spread of organism(s).
• It is important to educate the public, the pros and cons of ABHS. The importance of using moisturizer after using ABHS to prevent dry hand and skin damage.
Introduction
The COVID-19 pandemic posed unprecedented challenge to public health around the world. Universal vaccination is an effective measure to combat COVID-19, but public health infection control measures such as hand hygiene and mask for contact and respiratory control to prevent the spread of the virus has been the primary focus. The World Health Organization (WHO) and national disease control agencies emphasized the importance of hand hygiene to reduce the spread of the virus by frequent washing with soap and water after going to the bathroom, before eating or after coughing, sneezing or blowing one’s nose (1). When soap and water are not available sanitizing of non-visibly soiled hands with at least 60% alcohol-based agent is recommended (2). Each alcohol-based hand sanitizer (ABHS) formulation maybe effective to reduce or eliminate bacterial or viral load, it is commonly applied in the form of hand rub rinses, gel and foam (3), but it may impair skin barrier integrity and function, increasing the risk of hand dermatitis resulting local and/or systemic infections (4).
ABHS contains the alcohol ingredients isopropanol, ethanol or n-propanol or a mixture of the three agents (5). These ingredients have the anti-microbial ability to denature and coagulate proteins, thus causes the microbes to lose its protective lipid membrane, inhibition of its metabolism and induce lysis of the viral particle (1). The Center for Disease Control and Prevention recommends acceptable formulations to contain 80% (percent volume/volume) ethanol or 75% (percent volume/volume) isopropyl alcohol (2).
Our skin barrier contains a large proportion of stratum corneum, which composed of keratin and lipids (6). Under healthy conditions, the skin barrier colonized with various bacteria such as Staphylococcus epidermis, Staphylococcus aureus, Micrococcus spp., Propionibacterium spp. and Corynebacterium spp. which are not harmful to the human host (7,8). These bacteria may help to prevent the colonization of other pathogenic microbes by either competing with them for nutrients or stimulating the skin’s defense system and under exhibits low pathogenicity under normal healthy conditions (3). However, when the epidermis micro-environment is disrupted, by prolonged antibiotics use, frequent hand washing with alkaline soap and detergents, water with extreme temperatures, low humidity, repeated glove use, working in wet environment and rough paper towels these protective organisms can become virulent (9). A strict and frequent hand hygiene utilizing lipid-emulsifying detergents and lipid-dissolving alcohols can cause an acute loss of skin surface lipids, which depletes the corneum stratum protein, thereby decrease corneocyte cohesion and reduction of corneum water binding-capacity (10). The degraded skin barrier can increase transdermal water loss (TEWL), permits epidermal penetration of irritants, allergens and microbes, which propagates an inflammatory response resulting in hand dermatitis, which may contribute to local or systemic infection (10).
This is first reported case of a patient developed Staphylococcus lugdunensis (S. lugdunensis) induced aortic and mitral valve endocarditis, with excessive use of ABHS without moisturizer, compromised hand skin integrity. The patient required operative valves repair, veno-venous extracorporeal membrane oxygenation (VV-ECMO), dialysis and an intra-aortic balloon pump (IABP) while post-operation in the intensive care unit (ICU) for refractory hypoxia from severe congestive heart failure. We present this case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-35/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
A retired 75-year-old woman lives at home caring for her husband with dementia. She is a life-long non-smoker, drinks occasional alcohol, has no history of intravenous (IV) drug use, and no recent dental procedures. Her past medical history consisted of hypertension; hypothyroidism and left breast cancer diagnosed 21 years previously and treated with lumpectomy and radiation. During the COVID-19 pandemic, she maintained strict hand hygiene by applying frequently ABHS without moisturizer, which resulted her hands to be excessively dry, coupled with moderate skin erythema and cracks. Two weeks prior to her hospital admission January 13, 2022, family members and patient noted she was experiencing progressive generalized fatigue and shortness of breath with moderate exertion, bilateral lower leg edema, orthopnea and paroxysmal nocturnal dyspnea associated with fever and chills. She was admitted to the Cardiology Service at University Hospital, London Health Sciences Centre (LHSC), London, Ontario, Canada for presumptive Non-ST-Elevation Myocardial Infarction (NSTEMI) and decompensated heart failure. Her troponin was elevated at 39 ng/L, the electrocardiogram (EKG) showed normal sinus rhythm and non-specific ST changes, and the chest X-ray showed congestive heart failure. She denied experiencing any chest pain with exertion or at rest, as well as any localizing infectious symptoms such as urinary track symptoms, and cough or sputum production. Her home medication consisted of Amlodipine 5 mg, p.o. daily, Atenolol 50 mg, p.o. daily and Levothyroxine 88 mcg, p.o. daily. Her laboratory tests on admission showed Hbg 106 g/L, white blood cell (WBC) 16.7×109/L, platelets 248×109/L, sodium 134 mmol/L, potassium 4.1 mmol/L, chloride 97 mmol/L, bicarbonate 21 mmol/L, urea 9.3 mmol/L and creatinine 94 µmol/L. Blood cultures were also drawn.
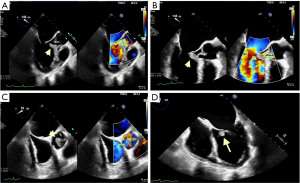
The patient was treated with loading clopidogrel 600 mg oral (p.o.), aspirin 81 mg oral (p.o.) daily, Ceftriaxone 2 g IV q24 h and low molecular weight heparin (LMWH) 5,000 units subcutaneous (SC) for deep vein thrombosis (DVT) prophylaxis. A transthoracic echocardiogram (TTE) on January 15, 2022 revealed left ventricular ejection fraction (LVEF) ≥70%. The left and right ventricle were normal in size, but a large 28×10 mm mass on the aortic valve was found prolapsing across the valve into the left ventricular outflow tract (LVOT) and associated with severe aortic regurgitation. No vegetation seen on the mitral valve but there was mild to moderate mitral regurgitation. A transesophageal echocardiogram (TEE) performed on January 18, 2022 found a 21×9 mm vegetation attached to the right coronary cusp of the aortic valve. The cusp prolapse was associated with severe aortic regurgitation and a non-mobile density, 7×4 mm, attached to the anterior mitral valve leaflet associated with moderate mitral regurgitation, Figure 1. A heart catheterization performed on January 19, 2022 revealed normal left main artery, 25% stenosis in the proximal left anterior descending artery, normal circumflex and right coronary arteries, and severe aortic regurgitation. The blood culture from January 13, 2022 reported as gram-positive cocci in clusters and the patient was started on ceftriaxone and vancomycin on January 14, 2022. On January 15, 2022, the final report showed that the two blood culture bottles were positive for S. lugdunensis susceptibility to Oxacillin/Cloxacillin and Trimethoprim/Sulfamethoxazole. The patient was switched to Cloxacillin for treatment of the S. lugdunensis. The urine culture showed pan-sensitive Escherichia coli (E. coli). The blood culture from January 17, 2022 showed one bottle out the two was positive for S. lugdunensis, and the blood culture from January 19, 2022 showed no growth in both bottles. The cardiac surgery service was consulted on January 15, 2022 and followed the patient closely in the Cardiac Coronary Unit (CCU). The patient continued to have decompensated heart failure with, despite optimal medical treatment. She was consented for urgent surgery on January 20, 2022.
A repeat TEE January 20, 2022 in the operating room revealed worsening mitral regurgitation and perforation in the anterior mitral valve. After cardiopulmonary bypass was initiated, the surgeon noted that the vegetation had destroyed the entire tricuspid aortic valve. A large abscess between the left and right commissure was found to burrow deep within the muscular septum into the right ventricular outflow tract, as well as the aorto-pulmonary window required extensive debridement and irrigation. After the aortic valve was excised in its entirety, the surgeon noted that the vegetation had spread to the anterior leaflet of the mitral valve and was eroding through the valve in two spots. The aortic valve was replaced with a 21 mm Edwards Magna bovine pericardial bio prosthesis; debridement and patch reconstruction of the left ventricular outflow track with a 5×4 cm autologous pericardial patch, followed by a complex mitral valve repair with a 2×2 cm anterior leaflet autologous pericardial patch, and a 34 mm Cosgrove band annuloplasty. The mean/peak gradient across the aortic valve was 15/35 mmHg, and 3/10 mmHg across the mitral valve. The total cross clamp time was 201 minutes. The patient had coagulopathy and required 12 units of packed red blood cells, 14 units of fresh frozen plasma, 4-pooled units of platelets and 500 mL 5% albumin.
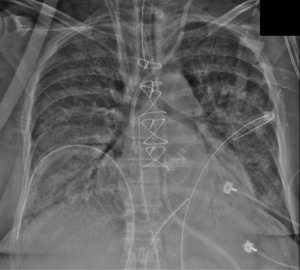
The patient transferred to the Cardiac Surgery Recovery Unit (CSRU) on norepinephrine 20 mcg/min, epinephrine 15 mcg/min, vasopressin 4 U/hour and cisatracurium 10 mg/hour. Her mechanical ventilation parameter indicated controlled mandatory ventilation on FiO2 100% and positive end-expiratory pressure (PEEP) 15 cmH2O. The arterial blood gas revealed pH 6.95, PO2 65 mmHg, PCO2 109 mmHg, bicarbonate 27.8 mmol/L, base excess (BE) −9.0 mmol/L and oxygen saturation 89%. Large volumes of pink frothy secretions aspirated from the endotracheal tube, and she was anuric. The chest X-ray showed bilateral white opacity consistent for pulmonary edema, Figure 2. The patient was placed on continuous venous-venous hemodialysis (CVVHD) for fluid removal. She continued to have poor oxygen saturation while on FiO2 100%, with arterial blood gas indicating pH 6.95, PO2 59 mmHg, PCO2 102 mmHg, bicarbonate 18.8 mmol/L, oxygen saturation 88% as her hypoxemia worsening her dosage of vasopressor and inotrope agents escalated. A bolus of methylene blue (at 1 mg/kg) given for persistent hypotension, which did not stabilize her blood pressure and a decision then was to put the patient on veno-venous extracorporeal membrane oxygenation (VV-ECMO) and intra-aortic balloon pump (IABP).
The pre-cannulation TEE January 20, 2020 showed hyperdynamic left ventricle (LV) with ejection fraction (EF) 80%, mild right ventricle dysfunction with no patent foramen ovale (PFO) or cardiac tamponade and the heart valves were functioning well. 1-liter of fluid and one unit of packed red blood cell (RBC) were given to correct intravascular volume depletion. VV-ECMO was initiated, with FiO2 100%, blood flow 4.3 L/minute, sweep gas flow 4 L/minute and rotation per minute (RPM) 3,585. The patient’s oxygen saturation dramatically improved to 100%. The native lung was maintained a tidal volume 210 mL, FiO2 40%, respiratory rate (RR) 6 breaths/minute, inspiratory pressure 10 cmH2O and PEEP 15 cmH2O. The patient’s hemodynamics stabilized within a couple of hours, norepinephrine infusion weaned to 15 mcg/minute, and vasopressin to 2.4 U/hour. Both epinephrine and cisatracurium infusion were discontinued. The arterial blood gas improved to pH 7.28, PO2 139 mmHg, PCO2 39 mmHg, bicarbonate 19.5 mmol/L, BE −7.9 mmol/L and arterial lactate 8.6 mmol/L. The patient continued on cloxacillin, and CVVHD with fluid removal and a negative balance achieved 1 to 1.5 liter/day. On day 3, hemodynamic improvement and decreased mechanical ventilation support. A trial sweep gas of zero passed successfully on ECMO with arterial blood gas of pH 7.35, PO2 95 mmHg, PCO2 40 mmHg, bicarbonate 23.4 mmol/L, BE −3.2 mmol/L, and lactate 1.2 mmol/L. VV-ECMO and IABP were subsequently decannulated without complications.
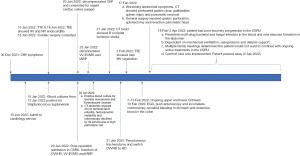
The patient’s subsequent clinical course complicated by a right occipital brain ischemic infarction, and suspected re-infection with mitral valve endocarditis on TEE, considered non-operable by cardiac surgery. This was followed by an upper gastrointestinal (GI) bleed due to a large stomach ulcer, detected by esophagogastroduodenoscopy (EGD) resulted gastric perforation, which required an open laparotomy, coupled with cecal bleeding and pancreatitis. She continued to have worsened renal failure, requiring CVVHD, and post laparotomy systemic resistant fungal infection with Candida glabrata (C. glabrata). The patient eventually succumbed to her illness and passed away 2 April 2022. A timeline of relevant events for this patient’s clinical course outlined in Figure 3.
Discussion
The COVID-19 pandemic resulted rigorous public health measures to minimize illness and overall death while limiting societal disruption. The global use of ABHS is one of the important means of controlling the transmission of the COVID-19 virus. The convenience and portability of hand sanitizers had led to their wide usage by 2020 and recommended by the WHO as an alternative hand hygiene measure (11). However, at the consumer level there are several safety concerns with ABHS includes its flammability, ingestion (accidental or intentional) and the potential adverse topical effects (11). Topically, ABHS has a low incident of adverse dermal effect with the exception of dry skin or more severely contact dermatitis (12). But, frequent application of skin irritants and allergens such as ABHS, without the protection of hand moisturizer or incorporation of emollients in it can compromise hand barrier and protective function and may result in local or systemic infection, in particular staphylococcus organisms (12). According to the patient’s family, she had a significant fear of contracting COVID-19 and spread to her elderly husband with dementia. She maintained strict discipline and zealous frequent application of ABHS without moisturizer resulted significant erythema and cracked skin of both hands which conceded systemic bacterial infection with S. lugdunensis.
Staphylococcus organisms rather coagulase-positive staphylococcus (CoPS), such as S. aureus that colonizes nearly 30% of the human skin and mucosa; or coagulase-negative staphylococcus (CoNS), such as Staphylococcus epidermidis (S. epidermidis) that colonizes nearly all human skin (9). This virulent bacterium produces a wide range of toxins implicated in the etiology of specific clinical manifestations, such as skin and soft tissue infection, necrotizing pneumonia and toxic shock syndrome (13). But culture positive CoNS, often indicating as contaminants and absence of virulence factors (13). However, the emergence of nosocomial and community pathogens with CoNS associated with the use of indwelling or implanted foreign bodies has become a key source of endogenous systemic infection (14) via sites, including blood, cardiac tissues, central nervous system and urinary tract (15). Particularly, CoNS can also act as a pathogen in the elderly and immunocompromised, including patients with diabetes, renal failure, or patients receiving chemotherapy (15). Our patient’s risk factor for CoNS infection is her age. She did not have any significant medical ailments, although had breast cancer 21 years ago treated with lumpectomy and radiation but clinically she was not immunocompromised.
S. lugdunensis one of CoNS specie organism initially described in 1988 (16). This organism is a part of the skin microbiome that is associated with the lower portion of the body, such as the perineum, the inguinal folds and the large toe nail; and upper extremities (17). Less frequently, S. lugdunensis can be found in the nasal cavity (18). The incidence of S. lugdunensis is lower than S. aureus and S. epidermidis (19). However, clinical comparison with other CoNS, S. lugdunensis virulence to be similar to that of S. aureus (20). Both organisms can produce clumping factors and/or heat stable DNase, and it has the same virulence factors (21). Furthermore, the morphological colony of S. lugdunensis on an agar plate can resemble S. aureus colonies (22). It can be differentiated from other CoNS by its ability to produce ornithine decarboxylase and pyrolidonyl arylamidase (PYR) (23). S. lugdunensis also causes severe skin and soft tissue infections, aggressive endocarditis, osteoarticular infections, vascular catheter related bacteremia and abscesses (24).
The majority of native valve endocarditis cases in non-IV drugs users, 20–35%, are caused by S. aureus (25). Among CoNS species, S. epidermidis causes the highest rates of native and prosthetic infective endocarditis (IE) (26). S. lugdunensis is the second most common pathogen associated with IE, as it cause 1% of aggressive IE cases with a mortality rate up to 40% (27). Published reports revealed that the clinical presentations of IE caused by CoNS were different from those caused by S. aureus (28). However, many reports indicated that S. lugdunensis was similar to S. aureus as both linked to IE with higher rates of complications and mortality (29). S. lugdunensis endocarditis most likely acquired in the community, usually without an identifiable source of infection (28). However, there are many case reports in the literature that reveal post-procedure development of S. lugdunensis infections that resulted in severe endocarditis, Table S1. S. lugdunensis endocarditis most frequently develop in male patients, older than 50 years old with a history of co-morbidity. 60% of the cases involves the aortic valve (30), and the rest involved both the aortic and mitral valves, such as in our case (28). CoNS invades prosthetic valves in the majority of cases, however, S. lugdunensis prosthetic valve involvement been reported by Liu et al. at 11.9%, and those patients usually carry a higher mortality due to underlying cardiac issues (28). S. lugdunensis native endocarditis carries an even higher mortality at 70% (28), and is aggressive and destructive towards left sided native valves associated with vegetation and/or abscess formation (31).
Fortunately, S. lugdunensis remains highly susceptible isoxazolyl penicillin and is not characteristic of therapy of other CoNS, such as S. epidermidis (32). Isolated case reports highlight the occurrence of resistance to streptomycin, erythromycin, ceftazidime, gentamycin, rifampin and ciprofloxacin (32). S. lugdunensis endocarditis often requires surgical intervention, as antibiotic therapy alone will not be sufficient. The incidence of surgical intervention for S. lugdunensis endocarditis is comparable to S. epidermidis, but higher incidence compared to S. aureus endocarditis (70% vs. 30%) and with a much higher mortality rate (28).
Conclusions
This is the first reported case of S. lugdunensis endocarditis induced by excessive use of ABHD without using moisturizer affecting the hand barrier function. S. lugdunensis bacteremia rarely acts as a contaminant or colonizing organism. Because of its virulence and expeditious tissue destructive characteristic, besides appropriate IV antibiotic therapy, early aggressive surgical intervention is necessary to limit the rapid progressive effect of the infection and cardiac dysfunction.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-35/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singh D, Joshi K, Samuel A, et al. Alcohol-based hand sanitisers as first line of defence against SARS-CoV-2: a review of biology, chemistry and formulations. Epidemiol Infect 2020;148:e229. [Crossref] [PubMed]
- U.S. Department of Health and Human Services. Food and Drug Adminstration, Centre for Drug Evaluation and Research (CDER). Policy for temporary compounding of certain alcohol-based hand sanitizer products during the public health emergency immediately in effect guidance for industry. 2020.
- Jing JLJ, Pei Yi T, Bose RJC, et al. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int J Environ Res Public Health 2020;17:3326. [Crossref] [PubMed]
- Montero-Vilchez T, Martinez-Lopez A, Cuenca-Barrales C, et al. Assessment of hand hygiene strategies on skin barrier function during COVID-19 pandemic: A randomized clinical trial. Contact Dermatitis 2022;86:276-85. [Crossref] [PubMed]
- Suchomel M, Eggers M, Maier S, et al. Evaluation of World Health Organization-Recommended Hand Hygiene Formulations. Emerg Infect Dis 2020;26:2064-8. [Crossref] [PubMed]
- Rundle CW, Presley CL, Militello M, et al. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol 2020;83:1730-7. [Crossref] [PubMed]
- Chiller K, Selkin BA, Murakawa GJ. Skin microflora and bacterial infections of the skin. J Investig Dermatol Symp Proc 2001;6:170-4. [Crossref] [PubMed]
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol 2008;158:442-55. [Crossref] [PubMed]
- Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9:244-53. [Crossref] [PubMed]
- Angelova-Fischer I, Dapic I, Hoek AK, et al. Skin barrier integrity and natural moisturising factor levels after cumulative dermal exposure to alkaline agents in atopic dermatitis. Acta Derm Venereol 2014;94:640-4. [Crossref] [PubMed]
- Abuga K, Nyamweya N. Alcohol-Based Hand Sanitizers in COVID-19 Prevention: A Multidimensional Perspective. Pharmacy (Basel) 2021;9:64. [Crossref] [PubMed]
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control 1998;26:513-21. [Crossref] [PubMed]
- Argemi X, Hansmann Y, Prola K, et al. Coagulase-Negative Staphylococci Pathogenomics. Int J Mol Sci 2019;20:1215. [Crossref] [PubMed]
- Becker K, Heilmann C, Peters G. Coagulase-negative staphylococci. Clin Microbiol Rev 2014;27:870-926. [Crossref] [PubMed]
- Natsis NE, Cohen PR. Coagulase-Negative Staphylococcus Skin and Soft Tissue Infections. Am J Clin Dermatol 2018;19:671-7. [Crossref] [PubMed]
- Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., Two Species from Human Clinical Specimens. International Journal of Systematic Bacteriology 1988;38:168-72. [Crossref]
- Heilbronner S, Foster TJ. Staphylococcus lugdunensis: a Skin Commensal with Invasive Pathogenic Potential. Clin Microbiol Rev 2021;34:e00205-20. [Crossref] [PubMed]
- Bieber L, Kahlmeter G. Staphylococcus lugdunensis in several niches of the normal skin flora. Clin Microbiol Infect 2010;16:385-8. [Crossref] [PubMed]
- Shin JH, Jung HJ, Lee HR, et al. Prevalence, identification, and antimicrobial susceptibility of Staphylococcus lugdunensis from various clinical specimens in Korea. Jpn J Infect Dis 2007;60:312-3. [PubMed]
- Parthasarathy S, Shah S, Raja Sager A, et al. Staphylococcus lugdunensis: Review of Epidemiology, Complications, and Treatment. Cureus 2020;12:e8801. [Crossref] [PubMed]
- Lambe DW Jr, Ferguson KP, Keplinger JL, et al. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can J Microbiol 1990;36:455-63. [Crossref] [PubMed]
- Hellbacher C, Törnqvist E, Söderquist B. Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect 2006;12:43-9. [Crossref] [PubMed]
- Sabe MA, Shrestha NK, Gordon S, et al. Staphylococcus lugdunensis: a rare but destructive cause of coagulase-negative staphylococcus infective endocarditis. Eur Heart J Acute Cardiovasc Care 2014;3:275-80. [Crossref] [PubMed]
- Argemi X, Prévost G, Riegel P, et al. VISLISI trial, a prospective clinical study allowing identification of a new metalloprotease and putative virulence factor from Staphylococcus lugdunensis. Clin Microbiol Infect 2017;23:334.e1-8. [Crossref] [PubMed]
- El-Ahdab F, Benjamin DK Jr, Wang A, et al. Risk of endocarditis among patients with prosthetic valves and Staphylococcus aureus bacteremia. Am J Med 2005;118:225-9. [Crossref] [PubMed]
- Monk AB, Boundy S, Chu VH, et al. Analysis of the genotype and virulence of Staphylococcus epidermidis isolates from patients with infective endocarditis. Infect Immun 2008;76:5127-32. [Crossref] [PubMed]
- Petti CA, Simmon KE, Miro JM, et al. Genotypic diversity of coagulase-negative staphylococci causing endocarditis: a global perspective. J Clin Microbiol 2008;46:1780-4. [Crossref] [PubMed]
- Liu PY, Huang YF, Tang CW, et al. Staphylococcus lugdunensis infective endocarditis: a literature review and analysis of risk factors. J Microbiol Immunol Infect 2010;43:478-84. [Crossref] [PubMed]
- Lin JF, Cheng CW, Kuo AJ, et al. Clinical experience and microbiologic characteristics of invasive Staphylococcus lugdunensis infection in a tertiary center in northern Taiwan. J Microbiol Immunol Infect 2015;48:406-12. [Crossref] [PubMed]
- Aldman MH, Rasmussen M, Olaison L, et al. Endocarditis due to Staphylococcus lugdunensis-a retrospective national registry-based study. Eur J Clin Microbiol Infect Dis 2021;40:1103-6. [Crossref] [PubMed]
- Kyaw H, Raju F, Shaikh AZ, et al. Staphylococcus Lugdunensis Endocarditis and Cerebrovascular Accident: A Systemic Review of Risk Factors and Clinical outcome. Cureus 2018;10:e2469. [Crossref] [PubMed]
- Taha L, Stegger M, Söderquist B. Staphylococcus lugdunensis: antimicrobial susceptibility and optimal treatment options. Eur J Clin Microbiol Infect Dis 2019;38:1449-55. [Crossref] [PubMed]
Cite this article as: Kao R, Alsaif N, Thain A, Kao V, Misener B, Chu M. Alcohol-based hand sanitizer’s effect on hand barrier function induced Staphylococcus lugdunensis aortic and mitral valve endocarditis: a case report. AME Med J 2023;8:26.

