
Management of chronic orchialgia: review of current clinical practice
Introduction
Chronic scrotal pain (CSP) or chronic testicular pain or chronic orchialgia (CO) has previously been defined as greater than 3 months of unilateral or bilateral scrotal pain interfering with daily life that ultimately leads to the pursuit of treatment (1). The pain is usually dull and may originate anywhere from the lower abdomen to the scrotum/glans penis. Urologists usually consider CSP when they rule out other causes like torsion, infection, testicular mass, etc. (2). CSP constitutes about 2.5–4.8% of all urology visits (3). It is believed to affect over 100,000 men annually (4). The condition can be challenging both for the patients and urologists and requires delicate care as the patients might be discouraged by the level of improvement in their pain. In this context, shared decision making, offering a holistic approach at an early stage, managing patient expectations by supportive counseling during this path is crucial for overall success. Although the most common etiology of CSP is idiopathic, there are many potential underlying causes that can be revealed with a thorough physical exam and history taking. These may include post-vasectomy pain syndrome (PVPS) (5), post-inguinal hernia repair (6), pain caused by trauma (7), pain after abdominal surgery (8), and radiation (9), etc. Treatment is mostly initiated with conservative modalities like medical management and neuro-modulation treatments [acupuncture (10), pelvic floor therapy (11), etc.]. Should such methods fail to improve the condition, more invasive options can be considered. Standard/targeted microsurgical denervation of the spermatic cord (SMDSC or TMDSC) (12,13), targeted nerve blocks (14), ultrasound-guided targeted peri spermatic cord/ilioinguinal cryoablation (UTC) (15-17), onabotulinumtoxin (Botox) application (Scrotox) (18), radical orchiectomy (19), and peripheric nerve stimulation (20) are examples to these surgical modalities. In this article, we present recent publications, surgical techniques and authors’ experience with possibly one of the largest CSP cohorts receiving these treatments and their outcomes in order to develop an approach strategy to aid the urologist in managing CSP cases.
Pathophysiology
Innervation of the spermatic cord and denervation of these nerves for CO was first described by Devine et al. (21). There is a complex relationship between ilio-hypogastric, ilio-inguinal, inferior hypogastric and genitofemoral nerve branches around the spermatic cord. Parekattil et al. previously described a “trifecta nerve complex” which could explain the pathophysiology of CSP (22). We (Parekattil et al.) biopsied spermatic cord samples from 57 men who underwent MDSC procedure for CSP versus a control group of 10 men who underwent cord surgery for varicocelectomies and radical orchiectomies. Tissue samples were obtained from mapped locations on the cord and evaluated by a pathologist. A median number of 25 small (<1 mm) nerve fibers were explored within the cord. Forty-eight of CSP cases (84%) had “Wallerian Degeneration” in at least one of the associated nerves vs. 20% of the controls (P<0.001). Mapping of these degenerations revealed 3 primary locations in decreasing order of nerve bulk: cremaster muscle fibers, the vasal sheath & perivasal tissue, and lipomatous structures on the posterior cord. Three human cadaveric dissections of the spermatic cord were also made to confirm the precise location of these nerve distributions corroborating the mapping. This is a novel study that proves a pathologic distinction of spermatic cord structure between CSP cases and healthy controls (22).
Wallerian degeneration in nerves has previously been linked with chronic pain in other areas of the body (23). This might also explain the beneficiary effects of ablation, ligation, or neuromodulation when treating CSP. It could also be the rationale for why targeted nerve block or spermatic cord block (SCB) prior to more cord-targeted therapies is predictive of good response to such treatments (24-26). Blocking the degenerated nerves during a targeted anesthetic block mostly induces temporary pain relief and/or reduction. This response is usually correlated to a successful outcome achieved with targeted modalities like MDSC, TMDSC, UTC, Scrotox, etc.
Pain characteristics
Typically, CSP patients present with distinctive pain distributions and features. Usually, the pain is described in testes by patients with tenderness in the epididymis revealed by physical examination. Neuropathic changes such as hypersensitivity or hyperalgesia in groin region may accompany these findings (27). The pain mostly waxes and wanes with severe pain episodes reaching 8 to 10 on a scale of 0–10. Validated, standard assessment tools to define pain in CSP patients have recently been developed (27,28). Polackwich et al.’s (28) tool is based on three fields to comprehend the patient’s experience of CSP. At first, the questionnaire consisted of 70 items that focused on: pain, urinary symptoms, location, sexual life, quality of life (QoL), and medical history. A cluster analysis was performed on responses given by the patients who were enrolled at two separate medical centers. One hundred and thirteen CSP patients completed the survey. Cluster analysis revealed a strict correlation between the QoL parameters and level of pain, pain occurring at night, pain that is burning type, pain extending to the cord and further down the groin, accompanying sexual dysfunction, and presence of premature ejaculation. CSP patients had a higher number of these symptoms. Factors affecting the QoL dramatically were burning-type of pain, pain occurring at night, pain extended to the groin & cord region, erectile dysfunction, and low sexual drive. In light of these, the group developed a Candidate Orchialgia Symptom Index (COSI) focusing on levels of pain, QoL and sexual symptoms. This selective approach (COSI) resulted in a more efficient, 12 question tool that was easy and simple to go through. COSI, also underwent external validation by being utilized for 170 CSP in 2 institutions (29). These data were analyzed methodologically for internal reliability, validity, consistency, floor and ceiling effects, responsiveness, and linear regression of all the questions (age, pain duration, etc.). The mean COSI score was 20±7.7 (range, 1–37), the pain score was 9.1±3.5 (range, 0–17), the sexual symptom sub-score was 1.8±1.5 (range, 0–5) and QoL sub-score was 9±4 (range, 0–15). Test/retest reliability was reported high, the retest total score was 21±7.9 and the intra-class correlation coefficient was 0.82. Internal consistency was 0.86 by Cronbach’s alpha. No total score floor or ceiling effects were observed. Construct validity revealed all parts contributed to a good fit model (P=0.001). COSI was not influenced by age, duration or prior surgeries. Lastly, the COSI responded to improvement post-therapy (mean: 13.5±9.8, P=0.00001). COSI can be considered a valid and clinically & statistically relevant tool to assess the symptom severity and the response to treatment in the follow-up period in cases with CSP.
Treatment algorithm for CSP
Below mentioned algorithm of evaluation methods, and treatment modalities for CSP is derived from a thorough review of recent (2000–2022) publications on National Library of Medicine (PubMed) and our experience over a couple of decades treating this complex condition. Figure 1 demonstrates the algorithm. The following sections of this review explain individual treatment options from the algorithm.
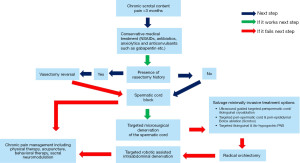
Conservative or non-surgical treatment options
Medical treatment of CSP has recently been reviewed by Starke et al. (30). Despite the fact that many urologists initially treat these patients with antibiotics, only 22% of CSP cases have an infectious cause, therefore antibiotics as a first-line treatment for CSP may not be ideal (31). Nonsteroidal anti-inflammatory drugs (NSAIDs) can be a considerable first-line option. Hot/cold packs, warm baths, as well as supportive undergarments, might also be beneficiary.
Low dose anxiolytics/tricyclic antidepressants (amitriptyline, etc.) can also be used and may offer up to 50% pain reduction (32,33). Antiepileptic medications with neuromodulating features (gabapentin, etc.) can also be considered and may provide a significant pain reduction in 80% of the cases (32). Some commercially available natural medications modulate neural pathways with a lesser side effect profile compared to gabapentin. One such example includes palmitic-acid mono-ethanol amide (PEA) (Canabrex, Theralogix, Rockville, MD, USA). A recent meta-analysis has shown that PEA significantly helps patients suffering from chronic pain (34).
Low vitamin B12 levels and testosterone levels can also be associated with CSP (35). Treating these deficiencies may ease the pain in some cases and can be considered a suitable conservative approach. Some CSP cases have accompanying bladder neck hypertrophy that contributes to their pain levels (36). In such cases, alpha-adrenergic inhibition might help. Lastly, acupuncture and pelvic floor therapy could help CSP cases reduce their pain (5,10).
Should these conservative therapies fail to provide sufficient pain relief, surgical interventions may be pursued. Patients who suffer from recurring pain despite these therapies, who are reluctant to keep taking medications/physical therapy sessions, or who seek more permanent solutions may also consent to surgical options.
Vasectomy reversal (vasovasostomy) for cases with PVPS
A distinct CSP subset is called PVPS. PVPS occurs in approximately 15% of the patients receiving vasectomy (37). It was found that the no-scalpel technique is superior to the scalpel technique in terms of PVPS rates (38). A good approach for CSP patients suffering from PVPS is a vasectomy reversal, particularly the ones who have congestive-type pain symptoms (scrotal pain that escalates post-ejaculation and is bilateral). Thus, in this cohort, the first invasive treatment following conservative modalities should be vasectomy reversal. Success rates with this procedure range between 69% to 100% (39-42). If the CSP patient with PVPS fails to respond to vasectomy reversal, targeted therapies like cord blocks and MDSC/TMDSC can also be pursued as if approaching a normal CSP patient (43). If these patients have nerve-type pain with more constant and unilateral features, MDSC/TMDSC may be a better option that should be suggested first (44).
Targeted SCB and ilioinguinal block
Pain linked to neural pathways as described above in the pathophysiology section should be relieved temporarily with targeted nerve blocks, and spermatic cord/ilioinguinal block. Therefore, the standard approach should be to perform these targeted blocks prior to more invasive treatments such as targeted surgical or ablative techniques (25). The period of pain relief after these blocks is typically short-term and the pain returns. Nevertheless, short response to these blocks provides a substantial predictive value of response to future-surgical modalities for CSP. A study by Benson et al. (25) discovered that a good response to a SCB was an independent predictor of response to MDSC in 74 men who received a cord block prior to MDSC. Our group’s study validated these findings (14) in a larger cohort. We retrospectively reviewed 1,261 MDSC cases (1,112 patients, 149 bilateral cases) between October 2008 and July 2019. We analyzed the correlation between patients’ temporary relief (>50% decrease in pain) following an SCB and their outcome post-MDSC. Final outcomes after MDSC were categorized to complete relief (CR), partial relief (PR) corresponding to >50% reduction, or no response (NR) corresponding to <50% reduction in pain. We used the validated pain impact questionnaire-6 (PIQ-6) and the visual analog score (VAS) systems to measure pre- and post-operative pain. The positive predictive value of a response to SCB to achieve a CR or PR after MDSC was 78% (CR alone 41%). The negative predictive value of a NR to SCB to achieve NR after MDSC was 57%. This work demonstrated that when a CSP patient does not respond to SCB, they will be less likely to benefit from a future MDSC. On the other hand, a good response to SCB significantly increases the chance of a significant reduction in pain from a future MDSC.
Technique for targeted spermatic cord/ilioinguinal block
The technique of SCB targets the trifecta complex. We initially palpate the vas deferens and place a needle next to it. We inject 5 cc of lidocaine, marcaine, and decadron mixture along and next to the vas deferens with a single insertion to avoid vessel injury. Peri-vasal tissue & peri-vasal sheath anesthesia were achieved with this injection. Then, the physician places a finger on the external inguinal ring. 32 cc of the local anesthetic mixture (15 cc 2% lidocaine, 15 cc 0.5% marcaine, and 2 cc 8 mg decadron) is applied to both sides of the finger to infuse the cremasteric layers of the spermatic cord as well as the outer areas. If the block is bilateral, half of the mixture is divided to each side. If it is unilateral, the whole volume is utilized on the corresponding side. Should the patient report pain in the groin area, or pain in the hip area, an ilioinguinal block can also be made. The ilioinguinal block is achieved by injecting 10 cc from the total 32 cc mixture into 2 cm inferior-medial to the anterior superior iliac spine (ASIS) and at a depth of 2 cm. Regardless of the block type, we usually perform cord blocks under intravenous (IV) sedation for both the patient’s comfort and the physician’s ease of access.
SMDSC/TMDSC
MDSC is a commonly practiced option for CSP. Various success rates (significant reduction or elimination of pain) ranging from 77–100% have been reported (13,43,45-49). The pathophysiology part describes the advantageous role of targeted therapies in CSP management. Targeting the degenerated nerves around the trifecta phenomenon possibly provides pain relief or reduction in CSP. MDSC contains ligation of all the components of the spermatic cord except the vessels and lymphatics. It’s considered aggressive, making the testicle susceptible to complications such as testicular atrophy, testicular loss, hydrocele, and/or lymphocele. Our group has come up with a targeted version (TMDSC) that ligates the trifecta complex only: first the cremasteric muscle, second the perivasal sheath (while preserving the vas deferens), and third the posterior lipomatous structures (13). We then assessed 772 TMDSC patients between October 2007 and July 2016. The pain was assessed pre-operatively and post-operatively using VAS and PIQ-6 scoring systems. At a 24-month median follow-up (range, 1–70 months), 718 cases (83%) showed significant (>50%) pain reduction, and 142 (17%) reported NR on VAS scoring. Of the 718 patients, 426 (49%) had CR and 292 (34%) had PR (>50% reduction in pain). Objective PIQ-6 scores showed a significant (>50%) reduction in pain in 67% of patients at 6 months, 68% at 1 year, 77% at 2 years, 86% at 3 years, and 83% at 4 years postoperatively (Table 1). This study illustrated that similar outcomes can be achieved with TMDSC with fewer morbidities. Another study by Kavoussi, also confirmed these results in 39 MDSC patients vs. 43 TMDSC patients (50). There was no difference in CR (66.7% vs. 69.8%, P=0.88), PR (17.9% vs. 23.3%, P=0.55), or NR rates (15.4% vs. 7.0%, P=0.22) between MDSC vs. TMDSC. Change in mean VAS score was also comparable (P=0.27). Operative time was significantly shorter in the TMDSC group (53 vs. 21 min, P=0.0001). They concluded that TMDSC can offer similar outcomes to SMDSC, less operative time, less challenging operation, and potentially less damage to the surrounding structures.
Table 1
| Follow-up period | Significant reduction in pain TMDSC group (n=772) | Significant reduction in pain UTC group (n=279) |
|---|---|---|
| 6-month | 67% | 60% |
| 1-year | 68% | 63% |
| 2-year | 77% | 65% |
| 3-year | 86% | 64% |
| 4-year | 83% | 59% |
TMDSC, targeted microsurgical denervation of the spermatic cord; UTC, ultrasound-guided targeted peri spermatic cord/ilioinguinal cryoablation.
Technique of TMDSC
Patient is left supine, induced anesthesia then prepped & draped in a standard fashion. The spermatic cord is dissected through a 2 cm subinguinal incision. The cord is, brought outside and secured over a tongue blade. A microsurgical platform (robot-assisted or microscopic) is brought. Ligation of the cremasteric layer is performed carefully. A micro-doppler (Vascular Technology Inc., Nashua, NH, USA) is used to locate and prevent damage to the testicular arteries. Vas deferens is then carefully dissected while preserving the deferential artery. The next targets for ligation are the vasal sheath and the perivasal structures. Lastly, the posterior spermatic cord lipomatous tissues are targeted. Majority of the cord is conserved as the internal spermatic sheath is not manipulated. A bioprotective material (Cygnus Wrap, Scendia Biologics Inc., Orlando, FL, USA) is wrapped around the cord to decrease post-operative scar formation (51,52). Spermatic cord is put back in the anatomic site and the incision is closed with 2-0 quilled suture (Quill, Surgical Specialties, Wyomissing, PA, USA) and hydrolyzed collagen powder (Cellerate, Scendia Biologics Inc.) and with 3-0 quilled suture for subcutaneous layer. Skin glue is applied after zipline closure of this layer.
UTC
For cases who do not respond to (T)MDSC therapy or for cases who want to pursue a less aggressive modality, UTC is considerable (15). Other studies have used this technology for similar scenarios targeting pudendal nerves and/or genitofemoral nerves with success (16,53). We performed 279 UTC cases (221 patients, 58 of whom are bilateral) between November 2012 and July 2016 who failed to respond to TMDSC before. We utilized a 16-gauge cryo-needle (Endocare, HealthTronics, Austin, TX, USA) for UTC. The needle was introduced at the level of the external inguinal ring, medial and lateral to the spermatic cord ablating the branches of genitofemoral, ilioinguinal and inferior hypogastric nerves. Pain levels were measured pre-op and post-op using the VAS and PIQ-6 (QualityMetric Inc., Lincoln, RI, USA). On a median 36-month follow-up (ranging 24 to 60 months), 75% of patients reported a significant reduction in pain (11% CR and 64% PR). PIQ-6 assessment revealed a significant reduction in 53% at 1 month, 55% at 3 months, 60% at 6 months, 63% at 1 year, 65% at 2 years, 64% at 3 years, 59% at 4 years and 64% at 5 years (Table 1). Complications were few and included two wound infections and four post-operative penile pain which resolved shortly after. Our study showed that UTC is a safe and feasible modality for salvage treatment of CSP refractory to TMDSC. Another good utilization of UTC may involve the treatment of residual groin/peri-incisional pain after TMDSC. Good response to targeted blocks in these areas potentiates more permanent relief achieved by UTC.
Technique of UTC
The patient is given IV sedation in the supine position, then prepped and draped. A cord block is performed as defined in previous sections. 1 mL of injectable amniotic-membrane derived fluid (Allogen, Scendia Biologics Inc.) is diluted in 3 cc saline and administered under ultrasound guidance to create a safe space between the medial side of spermatic cord and the lateral edge of the corporal body of the penis. This maneuver reduces the risk of irritation to penile sensory nerves and post-op penile pain. Then, the cryo-needle is mediolaterally inserted into the spermatic cord at the external inguinal ring level at a needle-depth of 3–4 cm. Real-time ultrasound guidance is used for this step. Once the needle is placed, cryoablation is performed for two cycles of 90 s on each side, with a passive thawing session in between the cycles. The needle is withdrawn once the ablation is concluded and an antibiotic cream is put on the insertion sites. Fluff dressings and jock support are used to reduce post-op scrotal swelling.
CSP patients who have a good response to MDSC/TMDSC for their testicular pain but keep having groin pain might benefit from UTC applied to the ilioinguinal nerve. For that purpose, with ultrasound guidance, the cryo-needle should be inserted 2 cm inferomedial to the ASIS. Cryoablation is performed in two 90-s sessions again with a passive thawing session after the first session.
UTC can also be utilized to reduce pain in peri incisional pain after TMDSC or MDSC (54). To achieve this, cryo-needle is inserted lengthways of the incision at 1 cm depth. The ablative session is made in a similar fashion, 90 s, two sessions, with a passive thaw session in between. Skin erythema and irritation at the ablation site may occur post-operatively. This can be managed with antibiotic gel two times a day for about 2 weeks.
Scrotox
Evidence on Botox for CSP is controversial (18,55). A study by Khambati et al. showed that Botox may provide pain relief for a period of 3 months in CSP patients (18). Out of 18 CSP patients that were enrolled in the study, 72% reported pain reduction at 1-month VAS scores (7.36 vs. 5.61, P<0.003). Additionally, Chronic Epididymitis Symptom Index (CESI) scores were also lower (22.19 vs. 19.25, P<0.04). At 3 months follow-up, 56% had sustained pain reduction on VAS scale (7.36 vs. 6.02, P<0.05). CESI score also remained reduced. Yet, at the 6-month follow-up, most patients returned to usual discomfort and pain. Our group retrospectively reviewed 44 patients who failed to respond to MDSC and subsequently received Scrotox between July 2013 and July 2016. 100 units of Botulinum toxin diluted with 10 cc saline was applied mediolaterally to the spermatic cord at external inguinal ring level as described before. Subjective VAS and the objective PIQ-6 tools were the primary outcomes. At 18 months median follow-up, 63% had a significant pain relief of VAS score. Assessment with PIQ-6 revealed pain relief in 27% of the patients at 6-month and 40% of the patients at 1-year. We concluded some of these patients may get a sustained pain reduction lasting about a year.
Scrotox is a feasible and safe option for CSP but requires re-application after approximately 3–12 months.
Technique for Scrotox
Scrotox application is follows the same “trifecta principle” as described before. IV sedation is induced to the patient. A cord block is performed as described above. 100 U of Botox is diluted in 10 cc saline. Mixture is applied mediolaterally to spermatic cord at external inguinal ring level. Should the patient have epididymal trigger pain and/or point tenderness, 2 cc of this mixture can be spared for around the epididymis. 8 cc is applied in the usual location. Real-time ultrasound guidance is used to prevent harm to any vessels.
Targeted ilioinguinal & ilio-hypogastric peripheral nerve stimulation (PNS)
PNS has previously been proven successful in CSP (56,57). Although this technique is usually performed by pain management specialists, recent technologies (Stimrouter, Bioness Inc., Valencia, CA, USA) allow urologists to implant an electrode easily along the ilioinguinal nerve easily. The novel stimulation electrode grants a whole different approach to managing CSP patients.
Radical orchiectomy (inguinal approach recommended)
Sometimes abovementioned therapies for CSP may fail and orchiectomy might be an option. Studies have shown an inguinal radical orchiectomy is preferable over a scrotal approach in terms of outcomes (1,58). This should only be an option after thorough discussions, going over all the pros, cons, and possible outcomes of such aggressive treatment. It is important to acknowledge that there’s a small risk of phantom pain and there is a chance that contralateral scrotal pain may occur post-radical orchiectomy. The physiology of these circumstances is obscure so it is suggested to pursue targeted therapies before this approach. Rate of success ranges from 20% to 75% (58).
Technique for radical orchiectomy (inguinal approach)
Patient is positioned supine, induced anesthesia then prepped & draped in a standard surgical fashion. An inguinal incision is made to expose the spermatic cord. Cord is then isolated and clamped. Testis is delivered into the inguinal incision without incising the tunica. The cord is then ligated and divided at the level of the internal ring and the contents are removed. Incision is closed to conclude the procedure.
Targeted robotic-assisted intra-abdominal denervation (TRAAD)
There aren’t many modalities for CSP cases failing TMDSC/MDSC or cases with continuous pain even post-orchiectomy. One approach for this challenging situation is TRAAD targeting inferior hypogastric nerve & genitofemoral nerve above the internal inguinal ring. The procedure, in nature, resembles tri-neurectomy procedure (59) where the ilioinguinal, iliohypogastric, and genitofemoral nerves are ligated. It is indicated in chronic abdominal/groin pain. Reported success range 70% to 80% (59,60). On the other hand, due to the pre-peritoneal location of these nerves and the ilioinguinal nerve’s function, sensory deficits in groin and scrotal dermatomes are expected. To have this sensory loss added to their persistent pain, can be debilitating for the patients. In order to overcome this, our group developed a TRAAD technique that preserves the ilioinguinal nerve and focuses on the inferior hypogastric and genitofemoral nerves. We reviewed 82 TRAAD patients between June 2009 to April 2019 retrospectively. We selected patients according to the following criteria: chronic (>3 months) groin pain, failed other steps such as TMDSC/MDSC, and cases with ongoing pain post-orchiectomy with unremarkable urologic workup. We utilized a robotic platform (DaVinci, Intuitive Surgical, Sunnyvale, CA, USA) for these procedures. The pain was assessed similarly to our other studies, with VAS and PIQ-6 tools. 71% (n=58) had a CR/PR in pain (>50% reduction). 33% (n=27) had CR. Mean follow-up was 71 (range, 4–120) months. There was one case reporting pain over assistant port and one reported bleeding from port site both of which required no active treatment. Two cases had leg pain and spasms postoperatively: one of which subsided on surveillance, and the other had persistent pain managed with painkillers. TRAAD seems like a feasible modality for challenging cases with persistent groin pain refractory to standard management and orchiectomy. Table 2 summarizes the success rates and indications of all the treatment methods that have been discussed so far.
Table 2
| Treatment modality | Indication | Consideration | Success rate |
|---|---|---|---|
| Conservative-anxiolytics/TCA | Failed NSAID treatment | Drug side effects | ~50% |
| Conservative-antiepileptic | Failed other medical treatments | Drug side effects | ~80% |
| Vasectomy reversal | Post-vasectomy pain syndrome | Alternative birth control methods | 69–100% |
| Targeted spermatic cord block | Prior to invasive treatments | Response is temporary | 78%* |
| Microsurgical denervation of the spermatic cord | First-line invasive modality, patients who respond to cord block | Salvage therapies should be considered if fails | 77–100% |
| Cryoablation | Failed MDSC, elective less aggressive treatment | Good for residual/incisional pain after MDSC | 59–75% |
| Radical orchiectomy | Last resort | Phantom pain | 20–75% |
| Abdominal denervation | Failed MDSC, post-orchiectomy pain | Sensory loss in groin and scrotal skin | 70–80% |
*, positive predictive value of targeted spermatic cord block for a successful MDSC. TCA, tricyclic antidepressants; NSAID, nonsteroidal anti-inflammatory drug; MDSC, microsurgical denervation of the spermatic cord.
Technique for TRAAD
In modified dorsal lithotomy position (same positioning for robotic-assisted laparoscopic prostatectomy), general anesthesia is induced. Trendelenburg is given. Patient is prepped & draped. Usually, 3 ports are placed consisting of a camera port and two instrument ports. A micro bipolar grasper is placed in the left arm and curved monopolar scissors in the other. The correct-side internal inguinal ring is located. Gonadal vessels are preserved but surrounding adventitia which contains the branches of genitofemoral nerve is ligated. Vas is also isolated and the perivasal structures containing the inferior hypogastric nerve plexus are ligated. Care is given not to damage the deferential artery during this stage. If the case has had an orchiectomy prior to this procedure, the TRAAD becomes basic: gonadal vessels and the cord canalizing into the internal inguinal ring are all ligated. The vas can also be ligated safely. That concludes the operation. Arms & ports are then removed followed by skin closure.
Chronic pain management
If all treatment options fail and the CSP patient is still suffering from pain, consulting a pain management specialist to collaborate for reserved options such as neuromodulation, medication, spinal blocks, etc. A psychiatric consultation can also be beneficial at this point. The key, as a urologist, is to be supportive and to maintain a hopeful mentality that makes future interventions possible. Physical therapy, acupuncture, behavioral therapy, and adjunctive medicine can also be initiated during any point of CSP management.
Strengths and limitations
Although authors share their experience with a large number of patients with long follow-up, the data are retrospectively reported. Prospectively designed, randomized studies and/or systematic reviews of the interventions described here are encouraged and warranted.
Conclusions
CSP is a challenging, bothersome disease to manage. Urologists should support and guide the patients throughout the whole process and navigate them through this wide spectrum of treatment modalities. It is crucial to keep the patient’s hope high and remind them not to give up. It is really important to collaborate with multi-specialty caregivers and the family to take care of the patient’s well-being as a whole. This article offers an evidence-based approach to CO for urologists in the hopes that treatment outcomes of this difficult condition improve.
Acknowledgments
The authors would like to thank all the patients who have helped us understand chronic orchialgia better over the years. Thanks to their trials and contributions, we have been allowed to shed light on the pathophysiology of this condition and generate treatment options to reduce their pain and suffering.
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.org/article/view/10.21037/amj-22-98/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.org/article/view/10.21037/amj-22-98/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davis BE, Noble MJ, Weigel JW, et al. Analysis and management of chronic testicular pain. J Urol 1990;143:936-9. [Crossref] [PubMed]
- Parekattil SJ, Ergun O, Gudeloglu A. Management of Chronic Orchialgia: Challenges and Solutions - The Current Standard of Care. Res Rep Urol 2020;12:199-210. [Crossref] [PubMed]
- Sigalos JT, Pastuszak AW. Chronic orchialgia: epidemiology, diagnosis and evaluation. Transl Androl Urol 2017;6:S37-43. [Crossref] [PubMed]
- Leslie SW, Sajjad H, Siref LE. Chronic Testicular Pain and Orchalgia. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing; 2023.
- Sinha V, Ramasamy R. Post-vasectomy pain syndrome: diagnosis, management and treatment options. Transl Androl Urol 2017;6:S44-7. [Crossref] [PubMed]
- Ducic I, Dellon AL. Testicular pain after inguinal hernia repair: an approach to resection of the genital branch of genitofemoral nerve. J Am Coll Surg 2004;198:181-4. [Crossref] [PubMed]
- Jørgensen SG, Öberg S, Rosenberg J. Treatment of longstanding groin pain: a systematic review. Hernia 2019;23:1035-44. [Crossref] [PubMed]
- Srivastava A, Kapoor R, Srivastava A, et al. Orchialgia after laproscopic renal surgery: a common problem with questionable etiology. Are there any predictors? World J Urol 2013;31:1153-7. [Crossref] [PubMed]
- Vistad I, Cvancarova M, Kristensen GB, et al. A study of chronic pelvic pain after radiotherapy in survivors of locally advanced cervical cancer. J Cancer Surviv 2011;5:208-16. [Crossref] [PubMed]
- Zhong LJ, Wang J, Ding XH. Therapeutic effect of electroacupuncture on chronic orchialgia. Zhongguo Zhen Jiu 2011;31:40-2. [PubMed]
- Farrell MR, Dugan SA, Levine LA. Physical therapy for chronic scrotal content pain with associated pelvic floor pain on digital rectal exam. Can J Urol 2016;23:8546-50. [PubMed]
- Ergun O, Gudeloglu A, Parekattil SJ. Robotic Surgery for Male Infertility and Chronic Scrotal Content Pain. J Endourol 2022;36:S48-60. [Crossref] [PubMed]
- Calixte N, Tojuola B, Kartal I, et al. Targeted Robotic Assisted Microsurgical Denervation of the Spermatic Cord for the Treatment of Chronic Orchialgia or Groin Pain: A Single Center, Large Series Review. J Urol 2018;199:1015-22. [Crossref] [PubMed]
- Parekattil S, Gudeloglu A, Ergun O, et al. PD58-06 What is the predictive value of a spermatic cord block prior to microsurgical denervation of the spermatic cord? J Urol 2020;203:e1202. [Crossref]
- Calixte N, Kartal IG, Tojuola B, et al. Salvage Ultrasound-guided Targeted Cryoablation of The Perispermatic Cord For Persistent Chronic Scrotal Content Pain After Microsurgical Denervation Of The Spermatic Cord. Urology 2019;130:181-5. [Crossref] [PubMed]
- Campos NA, Chiles JH, Plunkett AR. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician 2009;12:997-1000. [PubMed]
- Parekattil S, Gudeloglu A, Ergun O, et al. MP31-10 A cost effective office based technique for ultrasound guided peri-spermatic cord cryoablation for chronic scrotal content pain. J Urol 2021;206:e558-9. [Crossref]
- Khambati A, Lau S, Gordon A, et al. OnabotulinumtoxinA (Botox) nerve blocks provide durable pain relief for men with chronic scrotal pain: a pilot open-label trial. J Sex Med 2014;11:3072-7. [Crossref] [PubMed]
- Rönkä K, Vironen J, Kokki H, et al. Role of orchiectomy in severe testicular pain after inguinal hernia surgery: audit of the Finnish Patient Insurance Centre. Hernia 2015;19:53-9. [Crossref] [PubMed]
- Rosendal F, Moir L, de Pennington N, et al. Successful treatment of testicular pain with peripheral nerve stimulation of the cutaneous branch of the ilioinguinal and genital branch of the genitofemoral nerves. Neuromodulation 2013;16:121-4. [Crossref] [PubMed]
- Devine CJ Jr, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg 1978;70:149-51. [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Dubový P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat 2011;193:267-75. [Crossref] [PubMed]
- McLoughlin J, Kelley CJ. Study of the effectiveness of bupivicaine infiltration of the ilioinguinal nerve at the time of hernia repair for post-operative pain relief. Br J Clin Pract 1989;43:281-3. [Crossref] [PubMed]
- Benson JS, Abern MR, Larsen S, et al. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med 2013;10:876-82. [Crossref] [PubMed]
- Parekattil S, Gudeloglu A, Ergun O, et al. MP31-11 What is the predictive value of a spermatic cord block prior to targeted microsurgical denervation of the spermatic cord? J Urol 2021;206:e559. [Crossref]
- Aljumaily A, Al-Khazraji H, Gordon A, et al. Characteristics and Etiologies of Chronic Scrotal Pain: A Common but Poorly Understood Condition. Pain Res Manag 2017;2017:3829168. [Crossref] [PubMed]
- Polackwich AS, Arora HC, Li J, et al. Development of a clinically relevant symptom index to assess patients with chronic orchialgia/chronic scrotal content pain. Transl Androl Urol 2018;7:S163-8. [Crossref] [PubMed]
- Shoskes DA, Calixte N, Tadros N, et al. Validation of the Chronic Orchialgia Symptom Index for Men With Chronic Orchialgia/Chronic Scrotal Contents Pain. Urology 2018;119:39-43. [Crossref] [PubMed]
- Starke NR, Costabile RA. Medical management of chronic orchialgia. Transl Androl Urol 2017;6:S48-50. [Crossref] [PubMed]
- Strebel RT, Schmidt C, Beatrice J, et al. Chronic scrotal pain syndrome (CSPS): the widespread use of antibiotics is not justified. Andrology 2013;1:155-9. [Crossref] [PubMed]
- Sinclair AM, Miller B, Lee LK. Chronic orchialgia: consider gabapentin or nortriptyline before considering surgery. Int J Urol 2007;14:622-5. [Crossref] [PubMed]
- Ölçücü MT, Ölçücü N, Ölçücüoğlu E, et al. Response to duloxetine and gabapentin combination of a patient who has chronical orchialgia with bilateral tubular ectasia of rete testis and multiple epididymal cysts. Turk J Urol 2018;44:274-7. [Crossref] [PubMed]
- Artukoglu BB, Beyer C, Zuloff-Shani A, et al. Efficacy of Palmitoylethanolamide for Pain: A Meta-Analysis. Pain Physician 2017;20:353-62. [PubMed]
- Cui T, Terlecki R. Prevalence of Relative Deficiencies in Testosterone and Vitamin B12 Among Patients Referred for Chronic Orchialgia: Implications for Management. Am J Mens Health 2018;12:608-11. [Crossref] [PubMed]
- Hruz P, Danuser H, Studer UE, et al. Non-inflammatory chronic pelvic pain syndrome can be caused by bladder neck hypertrophy. Eur Urol 2003;44:106-10; discussion 110. [Crossref] [PubMed]
- Auyeung AB, Almejally A, Alsaggar F, et al. Incidence of Post-Vasectomy Pain: Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2020;17:1788. [Crossref] [PubMed]
- Skriver M, Skovsgaard F, Miskowiak J. Conventional or Li vasectomy: a questionnaire study. Br J Urol 1997;79:596-8. [Crossref] [PubMed]
- Horovitz D, Tjong V, Domes T, et al. Vasectomy reversal provides long-term pain relief for men with the post-vasectomy pain syndrome. J Urol 2012;187:613-7. [Crossref] [PubMed]
- Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol 1997;157:518-20. [Crossref] [PubMed]
- Smith-Harrison LI, Smith RP. Vasectomy reversal for post-vasectomy pain syndrome. Transl Androl Urol 2017;6:S10-3. [Crossref] [PubMed]
- Nangia AK, Myles JL, Thomas AJ JR. Vasectomy reversal for the post-vasectomy pain syndrome: a clinical and histological evaluation. J Urol 2000;164:1939-42. [Crossref] [PubMed]
- Tan WP, Levine LA. Micro-Denervation of the Spermatic Cord for Post-Vasectomy Pain Management. Sex Med Rev 2018;6:328-34. [Crossref] [PubMed]
- Tan WP, Levine LA. An overview of the management of post-vasectomy pain syndrome. Asian J Androl 2016;18:332-7. [Crossref] [PubMed]
- Tu XA, Gao Y, Zhang YD, et al. Microsurgical denervation of the spermatic cord for treatment of idiopathic chronic orchialgia. Chin Med J (Engl) 2012;125:2784-6. [PubMed]
- Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchialgia. J Urol 2001;165:1927-9. [Crossref] [PubMed]
- Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: long-term results from a single center. J Urol 2008;180:949-53. [Crossref] [PubMed]
- Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: a surgical alternative in the treatment of chronic orchialgia. J Urol 1996;155:1005-7. [Crossref] [PubMed]
- Sun HH, Tay KS, Jesse E, et al. Microsurgical Denervation of the Spermatic Cord: A Historical Perspective and Recent Developments. Sex Med Rev 2022;10:791-9. [Crossref]
- Kavoussi PK. Validation of targeted microsurgical spermatic cord denervation: comparison of outcomes to traditional complete microsurgical spermatic cord denervation. Asian J Androl 2019;21:319-23. [Crossref] [PubMed]
- Lemke A, Ferguson J, Gross K, et al. Transplantation of human amnion prevents recurring adhesions and ameliorates fibrosis in a rat model of sciatic nerve scarring. Acta Biomater 2018;66:335-49. [Crossref] [PubMed]
- Bourgeois M, Loisel F, Obert L, et al. Can the amniotic membrane be used to treat peripheral nerve defects? A review of literature. Hand Surg Rehabil 2019;38:223-32. [Crossref] [PubMed]
- Bittman RW, Behbahani K, Gonzalez F, et al. Interventional Cryoneurolysis: What Is the Same, What Is Different, What Is New? Semin Intervent Radiol 2019;36:374-80. [Crossref] [PubMed]
- Gabriel RA, Finneran JJ, Asokan D, et al. Ultrasound-Guided Percutaneous Cryoneurolysis for Acute Pain Management: A Case Report. A A Case Rep 2017;9:129-32. [Crossref] [PubMed]
- Dockray J, Aljumaily A, Lau S, et al. A Randomized, Double-Blind, Controlled Trial Shows that Onabotulinum Toxin A Nerve Blocks do Not Provide Improved Pain Control in Men with Chronic Scrotal Pain. J Urol 2020;203:767-72. [Crossref] [PubMed]
- Verrills P, Vivian D, Mitchell B, et al. Peripheral nerve field stimulation for chronic pain: 100 cases and review of the literature. Pain Med 2011;12:1395-405. [Crossref] [PubMed]
- Helm S, Shirsat N, Calodney A, et al. Peripheral Nerve Stimulation for Chronic Pain: A Systematic Review of Effectiveness and Safety. Pain Ther 2021;10:985-1002. [Crossref] [PubMed]
- Lowe G. Extirpative surgery for chronic orchialgia: is there a role? Transl Androl Urol 2017;6:S2-5. [Crossref] [PubMed]
- Karampinis I, Weiss J, Pilz L, et al. Transabdominal laparoscopic retroperitoneal neurectomy for chronic pain after inguinal hernia repair and appendicectomy -a matched-pair study. BMC Surg 2017;17:85. [Crossref] [PubMed]
- Gangopadhyay N, Pothula A, Yao A, et al. Retroperitoneal Approach for Ilioinguinal, Iliohypogastric, and Genitofemoral Neurectomies in the Treatment of Refractory Groin Pain After Inguinal Hernia Repair. Ann Plast Surg 2020;84:431-5. [Crossref] [PubMed]
Cite this article as: Ergun O, Gudeloglu A, Parekattil SJ. Management of chronic orchialgia: review of current clinical practice. AME Med J 2023;8:22.

