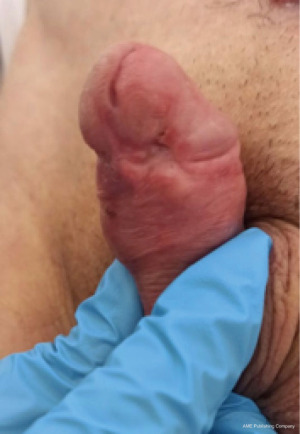
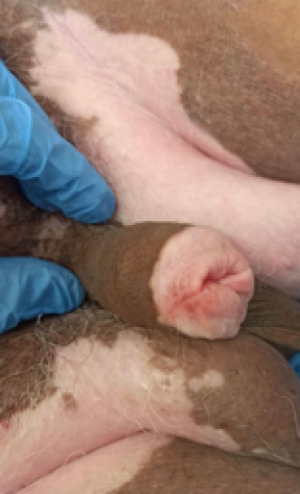
Penile preservation surgery in penile cancer
Introduction
Background
Penile cancer (PC) is rare in developed countries, with an estimated incidence of 1 in 100,000 men in the US and Europe. However, in other regions of the world, this rate is higher. The highest incidence has been observed in South America, Africa, and Asia (2.3–8.3 cases/100,000), and in certain countries, such as Uganda, PC is the most commonly diagnosed cancer in males (1). Recently, Maranhão, Brazil recorded one of the highest incidences of PC worldwide, predominated by locally advanced disease at the diagnosis (2).
A common histological subtype of PC is squamous cell carcinoma (SCC), with more frequent involvement of the distal regions of the penis—58% of the cases occur on the glans, compared with 16% on the foreskin and 9% on both the glans and foreskin. SCC commonly develops in the sixth and seventh decades of life. But, it can also affect young patients, a Brazilian survey reported that up to 38% of men with SCC were aged under 55 years at diagnosis (3,4).
Several risk factors for PC have been identified. There is a strong relationship between phimosis and SCC, likely due to chronic infections, although the carcinogenic role of smegma remains unknown. Other important risk factors are low socioeconomic status, poor personal hygiene, multiple sexual partners, and human papillomavirus (HPV) infection (4). Recently, sex with animals has been reported as an independent risk factor of PC (5).
Rationale and knowledge gap
Nearly 40% of men with PC are diagnose with localized disease, which has a 5-year overall survival of ~90% (6). Historically, the classical treatment for primary PC has been partial or total penectomy with perineal urethrostomy. Both of these surgical procedures, however, are mutilating and have a significantly negative impact on the patient’s quality of life, self-image, and sexual function. Although the cumulative 5-year recurrence-free rates of penile-sparing surgery are reported to be 82% in case series (CS) and 76.7% in non-randomized comparative studies (NRCSs) and the cumulative 5-year recurrence-free rates of amputative surgery are reported to be 83.9% in CS and 93.3% in NRCSs, the then-standard 2-cm surgical margins are no longer mandatory, and today, 5-mm margins are considered to be oncologically safe, rendering organ-sparing surgeries (OSSs) the standard surgical approach, whenever possible (7,8).
Objective
We aim to revisit and update the current knowledge on penile preservation surgery for PC. Ablative procedures, topical treatments, and irradiation modalities will also be addressed briefly.
Surgical techniques
Laser therapy
Laser therapy was first used for PC in the late 1960s with the ruby laser. This modality was derived from MASER (microwave amplification by stimulated emission of radiation) and was developed by Theodore Maiman (9). After decades of evolution and improvements, laser therapy is now used for small Ta, Tis, and T1 superficial tumors, yielding good aesthetic and functional results. However, local recurrence rates can reach up to 50%, occurring primarily in the first year, necessitating a restricted follow-up (10,11). Giant condylomas and papillary and warty tumors are superficially invasive neoplasms with low malignant potential that can also be treated by laser (7).
During this procedure, topical acetic acid, under visual magnification, can be applied to the surrounding skin to help identify satellite lesions that could be invisible to the naked eye (4).
A CO2 or Nd:YAG laser can be used; whereas the former is limited by its superficial depth of penetration (0.02–1 mm), the latter has a deeper penetration depth of 3 mm. In certain cases, the 2 lasers can be combined—the CO2 laser cuts better and can be used first to excise the lesion, followed by the Nd:YAG laser to coagulate the tumor bed (4).
Despite the functional advantages of laser therapy, pathological staging can be compromised due to thermal damage, rendering it difficult to determine the tumor staging precisely (9).
Schlenker et al. (10) retrospectively evaluated 54 patients who were treated with (Nd:YAG) laser: 11 with carcinoma in situ (Tis), 39 with T1, and 4 with T2 tumors. There was local recurrence in 16 patients (42%), but notably, there was no significant difference in recurrence rates in those with Tis or invasive penile carcinoma (11). However, for tumors stage ≥T2, the high rates of recurrences (32% to 100%) suggest that this method is unsuitable (12).
Mohs micrographic procedure
Mohs micrographic surgery (MMS) is a technique in which consecutive thin cross-sections of are lesion are resected, in association with intraoperative microscopic evaluation, until the surgical margins are negative. This approach allows one to identify subclinical lesions and effect maximum preservation of normal tissue. Some groups sharply debulk the tumor with a scalpel prior to removing the MMS layer (13,14). In the classical paper from 1985, Mohs and colleagues presented a series of 29 consecutive patients with SCC who were treated with this technique. The 5-year cure rate among the 25 determinate cases was 68% (15). In a more recent series, there was 1 recurrence among the 19 primary SCCs in situ that were treated with MMS, resulting in a cure rate of 94.7% (median follow-up 75 months). Of the 10 patients with primary invasive SCC, there was no recurrence (median follow-up 177 months) (13).
The success rates of MMS appear to be inversely proportional to PC lesion size. In a recent review of 43 patients who underwent MMS, only 1 patient experienced local recurrence at a median of 47 months of follow-up. Local recurrence rates for Ta, Tis, and T1 PCs were 0%, whereas that for T2 patients was 14% at 1 year (16).
Unfortunately, MMS is time-consuming and costly, with limited indications, and requires an experienced multidisciplinary team. Thus, this method has not been used widely in urology.
Wide local incision/circumcision
Circumcision may be appropriate for select patients with small, low-grade lesions in the distal foreskin or as an adjunct technique in other cases, providing better exposure of the glans and facilitating postoperative follow-up. In certain cases with larger lesions, there is a need to suture the foreskin directly to the glans. The estimated recurrence rate for this technique is 15% (17).
For small Ta/T1 lesions on the glans and penile shaft, wide local excision can be performed using a primary suture (Figure 1), foreskin flap, or extragenital split-thickness skin graft (STSG). It is important to use intraoperative frozen sections to achieve negative surgical margins.
Glans resurfacing
First described by Dr. Aivar Bracka for severe cases of lichen sclerosus of the glans, glans resurfacing can be used to excise premalignant and superficial lesions of the glans, such as in situ SCC that is refractory to other treatments (18,19).
This technique involves placing a tourniquet at the base of the penis and making circular incisions in the perimeatal area and coronal sulcus. Next, the glans is divided into 4 quadrants, and the epithelium and subepithelium are removed completely from the underlying corpus spongiosum, from the meatus to the coronal sulcus for each quadrant. Biopsies of the underlying tissue are necessary. The STSG is removed from the thigh region or another glabrous skin area with a dermatome and fixed in the exposed area of the glans with 5.0 absorbable sutures and separate stitches. A Foley catheter is placed for 5 days, and the patient is on strict bedrest for 2–5 days to prevent shearing of the skin graft, it is noteworthy that a variation of this technique, called tie-over dressing for graft application (TODGA), eliminates the necessity to maintain the patient in bedrest. Partial glans resurfacing can also be performed by adding a frozen biopsy to the edges of the resection (20-23).
This procedure is associated with rapid recovery, quick return to sensation of the glans, and good preservation of penile function (24). The oncological results should be analyzed with caution, considering that studies have included small samples; nevertheless, the technique has proven to be safe for well-selected cases. Shabbir et al. evaluated 25 patients with in situ SCC, reporting an overall local recurrence rate of 4% and no cases of progression (22).
The glans resurfacing technique usually results in cosmetic changes, due the difference in skin tone between the penile shaft and grafted area (18).
Glansectomy and reconstruction techniques
Glansectomy is indicated for T1 and T2 tumors that are confined to the glans. The procedure begins with a subcoronal circumferential incision in the shaft skin, down to the level of Buck’s fascia, after which the dorsal neurovascular structures are ligated. The surgical plane under the Buck’s fascia is developed; the glans is then dissected off the corporal distal extremities, and the urethra is divided, completing the excision. Frozen sections are obtained from the dorsal surface of the corporal bodies and urethra (25,26).
The simplest approach for reconstruction after glansectomy is urethral spatulation, followed by suturing of the penile skin at the midline and around the neomeatus. However, this technique does not yield the appearance of a neoglans (27). To obtain a better aesthetic result, other techniques can be added to reconstruct a neoglans.
STSG reconstruction
The STSG is a frequently used option. This technique begins with suturing of the skin 2 cm from the tip, leaving the corporal heads exposed, after which an STSG is taken from the lateral thigh to cover the corporal heads. The graft is secured with absorbable sutures, and a Foley catheter is left in place for 5 days. Care must be taken with the frozen section—if the tips of the corpora cavernosa are compromised, they should be resected (25). Beech et al. evaluated 12 patients after reconstruction with STSG, reporting a disease-free survival rate of 91.7% (11/12) at a median follow-up of 14 months. Standing voiding, erectile function, and satisfied cosmesis were preserved in all patients, and no graft-related complications occurred (28).
Autologous testicular tunica vaginalis graft
In 2018, an Austrian group published a case report, using an autologous testicular tunica vaginalis graft to recover the glans. This tool appears to be a more reproducible technique for urologists, considering that it does not require dermatomes to remove the thigh graft. However, more studies should be performed (29).
Reconstruction of neoglans with cavernous rotation
This interesting technique is used to create the shape of the glans. After glansectomy, the tips of the corpora cavernosa are separated from each other and from the urethral stump, after which they are rotated ventrally and sutured under the urethra, resulting in the appearance of a balanopreputial sulcus (Figure 2A-2C). A skin graft is sutured over the corpora and stapled urethral stump (30).
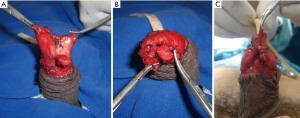
Distal urethral advance
This technique has been described to preserve penile sensitivity. After glansectomy, the penile urethra is separated completely from the corpora cavernosa to obtain a urethral stump that is at least 3 cm longer than the tips of the corpora. The urethra is then sectioned 3 cm ventrally and shaped to cover the cavernous apexes, everting the mucosa over the dorsal side of the penile albuginea. The urethral borders are fixed to the corpora cavernosa with absorbable sutures, and the skin of the penis is sutured 5 mm away from the neoglans. In 2007, Gulino et al. published a series of 14 patients who underwent glans reconstruction with urethral advance—8 after glansectomy and 6 after distal amputation. The tactile and thermal sensitivity of the neoglans was preserved in all patients, and no local disease recurrence was reported after a mean follow-up of 13 months (31).
In small tumors on the glans, it is possible to perform a partial glansectomy and use a foreskin flap to cover the defect (Figure 3A-3C). It is important that the tumor be sufficiently far from the urethral meatus, to avoid deviation of micturition (30).
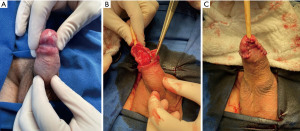
Oncological results after glansectomy
Albersen et al. retrospectively evaluated 117 patients after glansectomy. After a mean follow-up of 33 months, 12.8% of patients experienced local recurrence. Perineural invasion, carcinoma in situ, positive definitive margins, and the presence of high-grade SCC were predictive factors of recurrence (32).
A retrospective study that involved several international centers evaluated 410 patients who were treated with glansectomies. Only 31 patients experienced local recurrence, representing 7.6% of the study population. Fourteen patients (3.4%) developed regional recurrence, and 9 patients (2.2%) had distant recurrence. The authors concluded that glansectomy is a safe technique for SCC of the glans in appropriately selected invasive tumors (33).
The European Society of Urology considers glansectomy with reconstruction an option for distal pTa, pT1, and pT2 tumors (34).
Partial penectomy and procedures to enhance penile length
Classically, partial penectomy (Figure 4) is indicated when there is involvement of the corpora cavernosa. Additional goals of the procedure are to preserve the ability to void in a standing position and maintain sexual function. The surgery is similar to glansectomy, but the distal areas of the corpora cavernosa and urethra are resected together. Ideally, the urethral stump should be 1.5 cm longer than the corpora cavernosa. The corpora are closed transversally, and the penile skin is closed at the midline over the corporeal ends. The urethrostomy is performed by approximating the urethral stump to the adjacent penile skin with separate sutures (35).
Korkes et al. proposed a simple modification to the classical partial penectomy method to obtain better cosmetic and functional results: the parachute technique. In the updated version, the urethra is spatulated ventrally, and an inverted “V” skin flap with 0.5 cm of extension is sectioned ventrally. The suture is performed with Vicryl 4-0 in a “parachute” fashion, beginning from the ventral section of the urethra and the “V” flap, followed by “V” flap angles and then the dorsal portion of the penis. As a result, when the penis is flaccid and the skin is not retracted, the aspect is similar to the prepuce of an uncircumcised man (36).
In 2021, an Indian group developed a revised version of the “parachute technique” of Korkes et al., in which no V-shaped skin flap is created. After ventral urethral spatulation, the first skin suture is made on the apex of spatulation, followed by the lateral sides and finally on the dorsal side. Three patients were treated with this technique; at a mean follow-up of 8 months, all achieved good cosmesis and satisfactory functional preservation (37).
Several maneuvers have been described to enhance the length of the penile stump. Dividing the dorsal penile ligament and mobilizing the bodies proximally out of the pubic arch can lengthen the shaft by up to 2 cm; however, this technique can alter the angulation of the penile shaft during an erection (38). Ventral V-Y phalloplasty, which excises a segment of skin and the dartos of the penile/scrotal region, can help relieve tethering and create a new penoscrotal angle, lengthening the penis (7,39). Lipoaspiration of prepubic or penoscrotal fat has also been proposed (7).
Topical therapies and photodynamic therapy (PDT)
For all noninvasive PCs (e.g., carcinomas in situ and such eponymous tumors as Queyrat and Bowen disease), in 2016, the World Heal Organization recommended a single umbrella denomination: penile intraepithelial neoplasia (PeIN).
For the nonsurgical treatment of PeINs, beyond the laser (described above), several topical treatments have been historically applied, including 5-fluorouracil (5-FU), imiquimod (IQ), and PDT. The literature, varying widely and limited to small series, has generally reported a complete response rate of 40% to 50%, significant expenses for several weeks of therapy, and out-of-pocket expenses for drugs; local dermatological side effects and irritation can also develop, which are usually clinically manageable (40,41).
PDT has emerged recently as an effective treatment for PeINs; however, it is associated with postoperative pain and local erosions after application (41). Usually, PDT entails approximately 5 visits but is unavailable for urologists, being more commonly used by dedicated dermatological centers.
In 2020, Gao et al. reported 100% local control rates in China in 3 patients who underwent PDT with circumcision. The authors considered PDT to be controversial, but in this setting, they recommended it in conjunction with circumcision (42). It is unknown whether this combination is significantly superior to the exclusive postectomy, used in the last decades. In a large series of 315 patients who were treated by multidisciplinary urological and dermatological teams from several London hospitals, a group found that only 14.4% was treated successfully solely by cryotherapy or other topical treatments. The results on the use of topical treatment for PeIN remain “disappointing”, and 85.5% requires surgical procedures, with satisfactory results (43).
Radiotherapy
External beam radiotherapy (EBRT) has been used classically in PC to treat localized disease for patients who desire preservation of the phallus, but it has inferior local control rates compared with surgical treatment. When local recurrence occurs (20% to 40% of cases), salvage surgeries are effectives in most cases (44,45).
Penile tumors can be treated with interstitial brachytherapy (IB), surface mold plesiotherapy, and EBRT. Since the 1990s, EBRT has been replaced gradually by IB as the preferred irradiation modality for phallus preservation, given that its 5-year local control and cancer-specific survival rates (70% to 85% and 72% to 90%, respectively) are superior to those for EBRT for T1–T2 tumors (41% to 65% and 66% to 85%, respectively) (45,46).
The results of all radiotherapy modalities are volume-dependent and stage-dependent, beyond the inherent tissue radioresistance and radiosensitivity. Better oncological results and lower complication rates are achieved with IB for PC only for small lesions and for limited tumor radioactive lengths (<18 cm), tumor diameters (<4.0 cm), and tumoral volumes (<22 cc) (47).
It is important to emphasize that in addition to the previously described promising results involving radiation therapy, some recent series in brachytherapy as described by Pohanková and collaborators and Martz and collaborators demonstrate even better results in local control with rates close to or greater than 80% of penile preservation at 5 years in localized disease (T1–T2). Therefore, this well-established treatment modality is an excellent option when available (48,49).
Margins status, follow-up, and salvage procedures
As in other organ-sparing treatments in urological oncology, when OSS protocols are applied for PC patients, fundamental principles must be strictly followed to minimize and obtain secure intraoperative surgical margins, necessitating the use of frozen section biopsies. After such surgeries, patients must be monitored through rigorous long-term follow-up that promotes highly proactive and clinical suspicion of early detection of local recurrences. If necessary, salvage treatments must be as oncologically safe as primary radical procedures, without compromising survival rates (6,34).
Conclusions
OSS should be uses in PC whenever possible. Compared with classical penile amputations, OSSs are associated with a higher local recurrence rate (8). However, most of these recurrences can be treated with another OSS or amputation; thus, overall survival does not appear to be affected (50). In conclusion, when used properly, conservative techniques add significant benefits to quality of life but require strict follow-up and a collaborative patient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Penile Cancer”. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-25/coif). The series “Penile Cancer” was commissioned by the editorial office without any funding or sponsorship. SCZ served as the unpaid Guest Editor of the series. He also serves as an unpaid editorial board member of AME Medical Journal from June 2022 to May 2024. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures presented in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent for publication of this manuscript and accompanying images was obtained from patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Laversanne M, et al. Cancer Incidence in Five Continents: Inclusion criteria, highlights from Volume X and the global status of cancer registration. Int J Cancer 2015;137:2060-71. [Crossref] [PubMed]
- Coelho RWP, Pinho JD, Moreno JS, et al. Penile cancer in Maranhão, Northeast Brazil: the highest incidence globally? BMC Urol 2018;18:50. [Crossref] [PubMed]
- Fang A, Ferguson J. Penile Sparing Techniques For Penile Cancer. Postgrad Med 2020;132:42-51. [Crossref] [PubMed]
- Favorito LA, Nardi AC, Ronalsa M, et al. Epidemiologic study on penile cancer in Brazil. Int Braz J Urol 2008;34:587-91; discussion 591-3. [Crossref] [PubMed]
- Zequi Sde C, Guimarães GC, da Fonseca FP, et al. Sex with animals (SWA): behavioral characteristics and possible association with penile cancer. A multicenter study. J Sex Med 2012;9:1860-7. [Crossref] [PubMed]
- Thomas A, Necchi A, Muneer A, et al. Penile cancer. Nat Rev Dis Primers 2021;7:11. [Crossref] [PubMed]
- Kamel MH, Bissada N, Warford R, et al. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J Urol 2017;198:770-9. [Crossref] [PubMed]
- Sakalis VI, Campi R, Barreto L, et al. What Is the Most Effective Management of the Primary Tumor in Men with Invasive Penile Cancer: A Systematic Review of the Available Treatment Options and Their Outcomes. Eur Urol Open Sci 2022;40:58-94. [Crossref] [PubMed]
- Zarrabi A, Gross AJ. The evolution of lasers in urology. Ther Adv Urol 2011;3:81-9. [Crossref] [PubMed]
- Schlenker B, Tilki D, Seitz M, et al. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: a long-term follow-up. BJU Int 2010;106:786-90. [Crossref] [PubMed]
- Tang DH, Yan S, Ottenhof SR, et al. Laser ablation as monotherapy for penile squamous cell carcinoma: A multi-center cohort analysis. Urol Oncol 2018;36:147-52. [Crossref] [PubMed]
- Bandieramonte G, Colecchia M, Mariani L, et al. Peniscopically controlled CO2 laser excision for conservative treatment of in situ and T1 penile carcinoma: report on 224 patients. Eur Urol 2008;54:875-82. [Crossref] [PubMed]
- Machan M, Brodland D, Zitelli J. Penile Squamous Cell Carcinoma: Penis-Preserving Treatment With Mohs Micrographic Surgery. Dermatol Surg 2016;42:936-44. [Crossref] [PubMed]
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am 1992;19:291-304. [Crossref] [PubMed]
- Mohs FE, Snow SN, Messing EM, et al. Microscopically controlled surgery in the treatment of carcinoma of the penis. J Urol 1985;133:961-6. [Crossref] [PubMed]
- Alcalá NE, Reines KL, Merritt B, et al. Mohs microsurgery for localized penile carcinoma: 10 year retrospective review of local recurrence rates and surgical complications. Urol Oncol 2022;40:457.e1-7. [Crossref] [PubMed]
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol 2012;188:803-8. [Crossref] [PubMed]
- Alnajjar HM, Randhawa K, Muneer A. Localized disease: Types of reconstruction/plastic surgery techniques after glans resurfacing/glansectomy/partial/total penectomy. Curr Opin Urol 2020;30:213-7. [Crossref] [PubMed]
- Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int 2000;86:459-65. [Crossref] [PubMed]
- Malone PR, Thomas JS, Blick C. A tie-over dressing for graft application in distal penectomy and glans resurfacing: the TODGA technique. BJU Int 2011;107:836-40. [Crossref] [PubMed]
- Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and reconstruction of the glans penis. Eur Urol 2007;52:893-8. [Crossref] [PubMed]
- Shabbir M, Muneer A, Kalsi J, et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: surgical technique and outcomes. Eur Urol 2011;59:142-7. [Crossref] [PubMed]
- Pappas A, Katafigiotis I, Waterloos M, et al. Glans Resurfacing with Skin Graft for Penile Cancer: A Step-by-Step Video Presentation of the Technique and Review of the Literature. Biomed Res Int 2019;2019:5219048. [Crossref] [PubMed]
- Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int 2006;98:532-6. [Crossref] [PubMed]
- Smith Y, Hadway P, Biedrzycki O, et al. Reconstructive surgery for invasive squamous carcinoma of the glans penis. Eur Urol 2007;52:1179-85. [Crossref] [PubMed]
- Pietrzak P, Corbishley C, Watkin N. Organ-sparing surgery for invasive penile cancer: early follow-up data. BJU Int 2004;94:1253-7. [Crossref] [PubMed]
- Pettaway CA, Crook JM, Pagliaro LC. Campbell-Walsh-Wein Urology, Tumors of the Penis. Partin AW, Dmochowski RR. Editors. 12th ed. Elsevier; 2021.
- Beech BB, Chapman DW, Rourke KF. Clinical outcomes of glansectomy with split-thickness skin graft reconstruction for localized penile cancer. Can Urol Assoc J 2020;14:E482-6. [PubMed]
- Weibl P, Plank C, Hoelzel R, et al. Neo-glans reconstruction for penile cancer: Description of the primary technique using autologous testicular tunica vaginalis graft. Arab J Urol 2018;16:218-23. [Crossref] [PubMed]
- Pompeo AC, Zequi Sde C, Pompeo AS. Penile cancer: organ-sparing surgery. Curr Opin Urol 2015;25:121-8. [Crossref] [PubMed]
- Gulino G, Sasso F, Falabella R, et al. Distal urethral reconstruction of the glans for penile carcinoma: results of a novel technique at 1-year of followup. J Urol 2007;178:941-4. [Crossref] [PubMed]
- Albersen M, Parnham A, Joniau S, et al. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol Oncol 2018;36:141-6. [Crossref] [PubMed]
- Tang DH, Yan S, Ottenhof SR, et al. Glansectomy as Primary Management of Penile Squamous Cell Carcinoma: An International Study Collaboration. Urology 2017;109:140-4. [Crossref] [PubMed]
- EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam, the Netherlands 2022. ISBN 978-94-92671-16-5.
- Greenberg RE. Surgical management of carcinoma of the penis. Urol Clin North Am 2010;37:369-78. [Crossref] [PubMed]
- Korkes F, Neves-Neto OC, Wroclawski ML, et al. Parachute technique for partial penectomy. Int Braz J Urol 2010;36:198-201; discussion 201. [Crossref] [PubMed]
- Ranjan SK, Ghorai RP, Kumar S, et al. Modified "parachute technique" of partial penectomy: A penile preservation surgery for carcinoma penis. J Family Med Prim Care 2021;10:1054-6. [Crossref] [PubMed]
- Hegarty PK, Shabbir M, Hughes B, et al. Penile preserving surgery and surgical strategies to maximize penile form and function in penile cancer: recommendations from the United Kingdom experience. World J Urol 2009;27:179-87. [Crossref] [PubMed]
- Wallen JJ, Baumgarten AS, Kim T, et al. Optimizing penile length in patients undergoing partial penectomy for penile cancer: novel application of the ventral phalloplasty oncoplastic technique. Int Braz J Urol 2014;40:708-9. [Crossref] [PubMed]
- Filonenko E, Kaprin A, Alekseev B, et al. Own experience in treatment of patients with penile cancer using photodynamic therapy. Biomed Res Int 2015;2015:245080. [Crossref] [PubMed]
- Manjunath A, Brenton T, Wylie S, et al. Topical Therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl Androl Urol 2017;6:803-8. [Crossref] [PubMed]
- Gao X, Wu L, Chen M, et al. Photodynamic therapy combined with circumcision for the treatment of penile intraepithelial neoplasia. Photodiagnosis Photodyn Ther 2020;31:101894. [Crossref] [PubMed]
- Kravvas G, Ge L, Ng J, et al. The management of penile intraepithelial neoplasia (PeIN): clinical and histological features and treatment of 345 patients and a review of the literature. J Dermatolog Treat 2022;33:1047-62. [Crossref] [PubMed]
- Korzeniowski MA, Crook JM. Contemporary role of radiotherapy in the management of penile cancer. Transl Androl Urol 2017;6:855-67. [Crossref] [PubMed]
- Hasan S, Francis A, Hagenauer A, et al. The role of brachytherapy in organ preservation for penile cancer: A meta-analysis and review of the literature. Brachytherapy 2015;14:517-24. [Crossref] [PubMed]
- Azrif M, Logue JP, Swindell R, et al. External-beam radiotherapy in T1-2 N0 penile carcinoma. Clin Oncol (R Coll Radiol) 2006;18:320-5. [Crossref] [PubMed]
- de Crevoisier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX). Int J Radiat Oncol Biol Phys 2009;74:1150-6. [Crossref] [PubMed]
- Martz N, Bodokh Y, Gautier M, et al. High-dose rate brachytherapy in localized penile cancer: 5-Year clinical outcome analysis. Clin Transl Radiat Oncol 2021;27:89-95. [Crossref] [PubMed]
- Pohanková D, Sirák I, Vošmik M, et al. High-Dose-Rate Brachytherapy as an Organ-Sparing Treatment for Early Penile Cancer. Cancers (Basel) 2022;14:6248. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
Cite this article as: Santos VE, Pinto PR Neto, Zequi SC. Penile preservation surgery in penile cancer. AME Med J 2023;8:23.