
Techniques, outcomes, and complications of minimally invasive inguinal lymphadenectomy in cancer of the penis: an international clinical review
Introduction
Background
Invasive penile squamous cell carcinoma (SCC) typically metastasizes to the inguinal lymph nodes (ILN) first. Due to social stigma surrounding the topic and a lack of expertise among clinicians, up to 25% of men are diagnosed with advanced-stage disease at the time of presentation (1). Despite growing experience in managing penile cancer over the years, surgery remains the action toward of therapeutics. The involvement of the lymphatics located in the inguinal region is the most crucial prognostic factor (2-4).
Rationale and knowledge gap
It recommends bilateral inguinal lymph node dissection (ILND) for all patients, after management of their primary lesions, who are staged as intermediate (T1b) and high-risk tumors (T2 and T3) (5). Nonpalpable lymph nodes (LN) with low-risk disease can undergo observation, while bulky, palpable LN should receive neoadjuvant chemotherapy before ILND. Additionally, any patient with a positive LN on dynamic sentinel node biopsy (DSNB) should undergo ILND as well. Our updated description encompasses all four distinct inguinal lymphadenectomy (MIIL) techniques, surpassing the previous review. Instead of simply citing surgical options, this resource offers a thorough analysis of each technique, outlining its various aspects and presenting a step-by-step guide to help readers understand and choose from the available options (6-9). Despite evidence that patients undergoing ILND had a survival benefit incomplete adherence to the guidelines has been described, probably due to fear of overtreatment and complications (2,10,11).
Among individuals with inguinal nodes that cannot be detected through touch, it is critical for prognostic factors associated with a greater chance of inguinal metastasis to be thoroughly assessed. When these factors are present, prophylactic surgical resection of the inguinal LN has shown to increase survival (12). On the other hand, for patients with palpable and resectable ILN, complete cure is possible with or without adjuvant chemotherapy. When nodal ulceration or local skin invasion is noted, surgery can promote symptom palliation or avoid death due to femoral bleeding (2).
Objective
Understanding the endoscopic anatomy is crucial in order to achieve successful outcomes when performing surgery. Failure to do so may result in incomplete dissection and incomplete surgery. Research has shown that endoscopic surgery in the inguinal region is a viable choice for patients with minor inguinal conditions, resulting in significantly reduced rates of adverse events (13). Retrograde, the most commonly used technique, begins at the highest point of the femoral triangle and progresses toward the inguinal ligament at the top (13,14). This clinical review will focus on describing the MIIL techniques applicable for patients diagnosed with penile SCC.
Minimally invasive option for inguinal lymphadenectomy surgical assessment and treatment of regional lymph nodes
Anatomic background
Penile carcinoma spreads to inguinal lymph nodes first (superficially and then deeply) before developing into a distant metastatic illness (15). The boundaries of the femoral triangle are defined by the adductor longus muscle on the inner aspect, the sartorius muscle on the outer aspect, and the inguinal ligament on the upper aspect. The fascia lata serves as the anatomical barrier that distinguishes the superficial and deep sets of inguinal lymph nodes. The template guidelines for the dissection are influenced since superficial nodes are the first to be affected. The viability of the dissected skin flaps is contingent on the presence of anastomotic vessels situated within Camper’s fascia’s superficial adipose layer, which run parallel to the natural skin creases in a lateral-to-medial direction.
Inguinal endoscopic anatomy
Endoscopic video inguinal procedures are often seen as a suitable choice for Individuals with inguinal conditions of small magnitude. The retrograde approach, commencing at the distal apex of the femoral triangle and advancing proximally toward the inguinal ligament, is the prevailing technique (13,14). To achieve successful outcomes with this procedure, it is primordial to be aware of the anatomy of the endoscopic region. The first big anatomic markers to identify are the boundaries of skin, Camper’s, and Scarpa’s fascia using a minor surgical opening. After this, finger manipulation can be used to set the trocars. Insufflation with CO2 gas and examination with a blunt optic instrument allows for appropriate separation of the skin from underlying lymphatic and vascular structures. he most superficial aspect of the femoral triangle is demarcated by the sartorius muscle, which functions as a band. The adductor longus is the average boundary, and they are both easily identifiable endoscopically. The Saphena Magna or great saphenous vein traverses the farthest ends and can be identified in many patients, even if the lymph nodes are not distinguishable. A proximal assessment would lead to the identification of the fossa ovalis, also referred to as the saphenous hiatus, which is the location of the accessory saphenous vein and other tributaries of the great saphenous vein.
One should be careful when resecting around the saphenous-femoral junction, as most hubs are located above this intersection. These hubs can be identified by their brown or green tinge. Preoperative ultrasound can be beneficial to examine the skin in the area of the most prominent hubs. If fluorescence is available, minimally invasive resection can be used to remove any doubtful hubs.
The femoral triangle is recognized when the fascial lata of the thigh is segmented over the femoral conduit’s beat. This procedure should expose the profound inguinal nodes and encompass all nodal and areolar tissue that lies adjacent to the femoral vein and runs parallel to the adductor longus muscle. The excision ought to persist until the discovery of Cloquet’s node, which is a proximal lymph node located within the femoral vessel.
The following procedures are all performed while the patient is under general anesthesia. Standard prophylactic antibiotics administered as single dose (second generation cephalosporin). Elastic stockings are employed for the prevention of deep vein thrombosis (DVT), while infection prophylaxis is achieved through the use of appropriate measures. The main instruments required with are: 30° laparoscopy optic, extraction bag, bipolar forceps and 5 mm endo-clip, monopolar scissors Ligasure™ (Covidien Surgical®, Minneapolis, MN, USA) vascular sealant.
Surgical techniques
Video endoscopic inguinal lymphadenectomy (VEIL)-conventional
The leg and thigh are flexed to expose the femoral triangle, which is marked with ink on the skin. Afterward, the leg is stretched out and fastened to the table, causing a slight abduction and external rotation of the thigh. A monitor displaying video is placed in front of the surgical area, near the patient’s pelvis.
To begin the procedure, a 1.5 cm incision is created 2 cm from the vertex of the femoral triangle, extending through the subcutaneous tissue until reaching Scarpa’s fascia. Additionally, a second 1 cm incision is made 2 cm above and 6 cm towards the middle from the initial incision, which serves as an entry point for a 10 mm port. By means of this approach, it is possible to locate the path of the saphenous vein. Subsequently, a laterally symmetric port with a diameter of 5 mm is inserted to facilitate the use of graspers, dissection tweezers, and scissors. A 10 mm Hasson trocar should be used as the opening port, with a 0-degree optic inserted at the same port. The tweezers of the harmonic scalpel and the clipper should be introduced at the medial port. The surgeon and camera operator are positioned to the side of the surgical site. CO2 is insufflated at a pressure of 15 mmHg to create a working area, which is then maintained at 5–10 mmHg throughout the surgery. Transillumination is employed to help determine the direction of the dissection, allowing for a more precise surgical procedure.
We use a harmonic scalpel to perform separating the skin flap in a retrograde manner, which is a key part of the procedure’s success. We begin by separating the skin from the fibro-areolar tissue that anchors the superficial lymph nodes, extending to the fascia of the external oblique muscle on the upper aspect. Next, we begin dissecting the critical structures, keeping within the confines of the medial long adductor muscle and its fascia, the lateral sartorius muscle and its fascia, and the inguinal ligament at the superior aspect. During this, we should keep an eye out for any branches that may be connected to the femoral nerve and must be preserved. Following this, we can trace the long saphenous vein and perform a craniotomy to reach the oval fossa.
After locating the femoral artery and opening the femoral vein sheath, we can establish the outer limit of our dissection, allowing us to access the deep cervical lymph nodes. It is currently necessary to control both the femoral artery and vein using 1–2 branch clips. To begin, tie off the fibroareolar tissue at the distal end of the femoral triangle vertex, then employ a harmonic scalpel to dissect it. Careful handling of the specimen near the veins is essential to prevent any vascular damage during dissection and release of the lymph nodes superior to the femoral region Similar to the conventional technique, the femoral veins should be skeletonized, and all local lymphatic tissue should be excised.
The long saphenous vein should be distally ligated with clips. Most branches can be controlled with a harmonic scalpel, but larger branches need to be ligated with metallic clips. The opening of the femoral vein should be meticulously dissected and secured with polymer clips, if possible. Free the specimen on the medial side of the long saphenous vein and ligate the proximal segment of the lymph nodes in the deep region of the femoral canal using clips. Once complete, the endoscope view should show that all tissue of the region has been fully resected.
To facilitate removal through the 15 mm incision, a bag should be used to contain large surgical specimens. For vacuum drainage, a 5 mm orifice should be utilized, and larger incisions should be sutured upon completion (Figure 1).
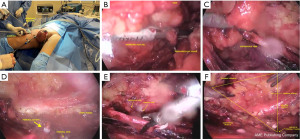
Lateral access VEIL (L-VEIL)
The patient is positioned in a supine posture on a split-leg table with legs spread apart. The affected leg is then abducted and slightly flexed at the knee. On the opposite side of the patient’s body, the scrub nurse stands while the video system is positioned at waist level on the opposite side of the operated limb. Prior to commencing the procedure, marking of the inguinal ligament, anterior superior iliac spine, and femoral triangle is carried out during the preoperative phase. IV antibiotic prophylaxis is also recommended. During the operation, the surgeon and the assistant stand lateral to the operative leg on the same side, so there is no need for position exchange. The 3-incision superolateral inguinal approach is used for L-VEIL.
A 1.5 cm vertical incision is made situated 6 cm beneath the anterior superior iliac spine. Via this incision, blunt dissection under Scarpa’s fascia is made using scissors and finger technique. A 12 mm trocar is inserted to accommodate a 30° laparoscope lens, with narrowing of the incision to avoid leakage. A second incision was made 3 cm below and 1 cm medially to the first port, to place a 12 mm trocar. This will be the main working port accepting the harmonic scalpel® (Ethicon, CA, USA)/Ligasure® (Covidien, MA, USA) alternatively, for the clip applier. The last 5 mm port is placed 3 cm above; 1 cm medially to the first port (at the level of vertex of femoral triangle). Graspers, scissors, or a dissection device can be used in this entry. The working area was infused with CO2 at 12 mmHg to achieve rapid space distension. Thereafter, to prevent the development of emphysema, CO2 pressure was maintained at 7–9 mmHg throughout the procedure. By trans-illumination, the dissection area’s progression towards the cavity can be efficiently tracked and monitored for proper orientation.
Using a harmonic scalpel or Ligasure, dissection is performed at the appropriate plane below Scarpa’s and Camper’s fascia until visualization of the external oblique aponeurosis occurs. This allows for removal of all superficial lymphatic tissue. The adductor longus muscle medially, the Sartorius muscle laterally, and the inguinal ligament superiorly are key landmarks that must be clearly visible during the procedure. Femoral nerve branches should be identified and preserved. Subsequently, the femoral artery is located at the femoral triangle, and the fascia is opened along its extension. Later, the femoral vein is identified, and the Saphenous vein is dissected and preserved whenever possible or ligated after clipping at Saphenofemoral junction.
Following that, clips are utilized to ligate the tributaries of the Saphenous vein, such as the superficial circumflex iliac, superficial epigastric, superficial external pudendal, as well as superficial lateral and medial cutaneous veins. The femoral canal is dissected until the pectineus muscle is visible in order to fully retrieve the nodes and expose Cloquet’s lymph nodes. Subsequently, clips are employed to isolate the dissected node-bearing tissue at the femoral apex. Depending on the size of the specimen, an incision of 1.5 or 2–2.5 cm is made in order to remove the specimen using a retrieval bag. Next, a suction drain is inserted via the lower port, and the incisions of the ports are closed. Following the procedure, an elastic compression bandage is applied from the lower leg to the thigh, and rehabilitation is promoted (Figure 2).
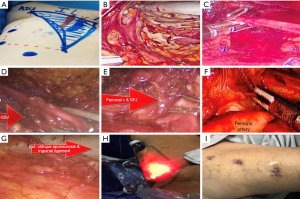
Hypogastric access VEIL (H-VEIL)
This access is ideal for patients that need inguinal and pelvic simultaneous lymphadenectomy, named as PISA (16). The patient is lying down on their back with their lower limbs apart. Delineating the Scarpa triangle in situ on the skin bilaterally is needed when performing an ILND. Blunt dissection is employed to establish a subcutaneous abdominal workspace, starting at the umbilicus and progressing caudally towards the targeted inguinal ligament. Afterwards, a dilating trocar-balloon is inserted. Three trocars are then placed: An 11 mm trocar is positioned at the optic access site, while two 5 mm trocars are placed at the midpoint of the lines joining the umbilicus with the pubic symphysis and the anterior-superior iliac spine, forming a triangle that points towards the inguinal ligament. Advanced balloon fixation trocars are recommended, and it is advisable to commence with low-pressure CO2 insufflation of 15 mmHg during trocar placement. After the completion of trocar placement, the pressure can be lowered to 10 mmHg. This approach reduces the likelihood of subcutaneous emphysema formation during the procedure.
Transillumination and manual visual inspection are utilized to establish a dissection plane between Camper’s and Scarpa’s fascia, commencing from the hypogastric area and progressing up to the inguinal ligament, which is the highest point for inguinal lymph node dissection The dissection advances downward with the aid of Ligasure™ vascular sealant, and the procedure proceeds once the boundary of the falciform process of the fascia lata and the saphenous opening (fossa ovalis) in the midline, as well as the medial edge of the Sartorius muscle on the lateral side, have been identified the long adductor muscle’s medial and lateral borders are exposed. When performing the superficial inguinal lymph node dissection, the saphenous vein should be preserved and as many of the venous tributaries as possible should be retained to avoid any loss of skin blood supply, until they reach the bottom of Scarpa’s triangle. Following that, dissection of the deep inguinal nodes is carried out by entering the saphenous opening and carefully excising the surrounding tissue from both femoral vessels. To prevent postoperative lymphorrhea, it is crucial to utilize both bipolar energy and surgical clips during this stage. The laparoscopic extraction bag, along with an 11 mm trocar, is used to completely remove the surgical specimen with a surgical drain is put in the wound to help it heal, and is left there until it has healed. The contralateral inguinal lymph node dissection (LND) can be performed using the 11 and 5 mm trocars already positioned in the midline. An extra trocar must be inserted at the midpoint between the umbilicus and the anterior-superior iliac spine on the opposite side, in addition to the 11 and 5 mm trocars already present in the midline. The patient is placed in a 30-degree Trendelenburg position, and the trocars used for the initial ILND are repositioned within the abdomen through the same incisions. An extended pelvic lymph node dissection is then performed. After the procedure, the surgical specimen is extracted through the 11 mm trocar, and a drainage tube is placed at the surgical site to prevent any fluid buildup (Figure 3).
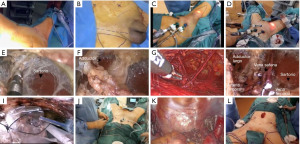
Robotic assisted VEIL (R-VEIL)
To perform a bilateral groin dissection, the patient is positioned in a low lithotomy position without altering the robot’s position. For a dissection on the right side, the assistant will stand lateral to the patient’s right leg, and for the left side, the assistant will stand in-between the patient’s legs. Once the inguinal and groin region has been prepared and draped, a sterile Foley catheter is inserted. The skin surface is marked with accurate landmarks to form an inverted triangle, with the base consisting of a line that connects the pubic tubercle and the anterior superior iliac spine, following the path of the inguinal ligament. The sartorius muscle forms the lateral boundary, extending towards the apex, while the adductor longus muscle forms the medial boundary, also pointing towards the apex. These markings serve as guides for trocar placement and dissection extent determination.
A 2-cm skin incision is created 3 cm inferior to the inferior aspect of the femoral triangle, located 2.5 cm below the inguinal ligament. Following that, the layer of Scarpa’s fascia, a white subcutaneous tissue, is identified. Using sweeping finger dissection, the potential space beneath the fascia is dissected, producing two skin flaps at the triangle’s apex in both directions. Afterwards, two 8-mm robotic ports are inserted using finger-guided techniques, one on the lateral side and one on the medial side. Using a 12-mm origin balloon port trocar (Origin Medsystems, Menlo Park, CA, USA), a subcutaneous workspace is created by insufflating the area at 25 mmHg for 10 minutes. Subsequently, this will enable the endoscope to move smoothly and produce a subcutaneous flap above Scarpa’s fascia. The CO2 insufflation pressure is decreased to 15 mmHg, followed by the insertion of a 0-degree 10-mm lens. An additional 10-mm assistant port is placed between the camera and the primary 8-mm working port on the assistant side. For the first operation, the robot is located 45 degrees away on the opposite side (right side) whereas for the second procedure, The robot is positioned on the ipsilateral side of the patient (left side). Delicate dissection of the membranous and lymphatic tissue deep to Camper’s fascia can be performed using a bipolar Maryland or PK forceps in the left robotic arm and a monopolar scissors in the right arm. It is important to ensure that the anterior working space is fully developed up to the inguinal ligament. The inguinal ligament can be easily identified by its transverse structure and white fibers, which mark the upper limit of the dissection. Care should be taken to achieve this. The superior boundary should be the inguinal ligament, while the lateral and medial boundaries should be the sartorius muscle and adductor longus muscle, respectively. Many patients can have their saphenous vein spared, and small branches of the femoral artery and vein may need to be clipped or divided. To help locate the sartorius and adductor longus muscles, look for their corresponding fascia in the previously skin markings. The medial location of the spermatic cord is observed, afterwards. Identification of the fascia lata is obvious when reddish muscular fibers are present. The nodal tissue can be carefully dissected using blunt techniques, rolling it inward from both sides until the inferior apex of the packet is reached. The saphenous vein can be identified as it crosses the medial border of the dissection near the apex of the femoral triangle. Following the vein, one can trace it back to its junction with the superficial femoral vein at the fossa ovalis. Blunt and sharp dissection is then used to carefully detach the packet from the fascia lata superiorly.
The packet is held with the nondominant hand while the dominant hand uses monopolar scissors to initiate the dissection. Then, the fossa ovalis should be reached, and then the packet should be cut away at its upper-lateral and upper-middle borders, decreasing the packet’s size and detaching it from the inguinal ligament. The packet is ultimately separated from the inguinal ligament in the lower region as the superficial and deep planes of dissection converge.
Whenever feasible, it is crucial to spare the saphenous vein to minimize the risk of lymphedema after the surgery. Once the nodal packet has been dissected circumferentially except for its attachment to the saphenous arch, it is important to clip any venous tributaries and identify the nearby pulsations of the femoral artery as a landmark. To release the packet from the saphenous vein, it can be attempted; if not possible, however, weak clips should be used to ligate the vein in the saphenous arch.
After the camera trocar incision has been extended, the specimen should be removed in a specimen retrieval bag. Frozen section results will be used to decide if a deep ipsilateral dissection is needed. During the wait for the pathology report, the working space on the opposite leg can be prepared. The CO2 space is reestablished for a deep inguinal node dissection. To reach the saphenofemoral junction, the fascia lata medial to the saphenous arch needs to be incised. The dissection in an inferomedial direction should continue around the femoral vein until the deep inguinal nodes are removed. To ensure complete retrieval of all nodes, the dissection should continue until the pectineus muscle is visible, which is at the level of the femoral canal. The fascia lata medial to the saphenous arch should be opened in order to reach the saphenofemoral junction (Figure 4).
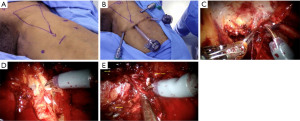
Once the desired amount of lymphadenectomy has been performed, to ensure hemostasis has been achieved, the insufflation pressure should be lowered to 5 mmHg. This step is especially necessary to avoid potential infection-causing lymphocele and hematoma formations. Additionally, a vacuum drain should be inserted and positioned at the base of the lymphadenectomy site, so that fluid can move downward towards the drain while the patient is standing up. Lastly, standard closing procedures should be applied to the trocar incisions.
Post operative care
Low-weight heparin (3,500 IU/day of sodium bemiparin) begins within 24 hours of the surgery and is continued for a period of 30 days. It is encouraged to begin ambulating and consuming liquids orally within 12 hours. Drainages are taken out when the daily drainage is less than 50cc. Discharge is generally planned for the first postoperative day, when all drainages have been removed.
Complications of inguinal lymphadenectomy
The estimated perioperative morbidity rate for ILND is high, with figures varying from 3% to 97% (17). In recent years, surgeons have strived to reduce morbidity in surgery without compromising oncological principles, resulting the development of more compact/narrow resection patterns, the conservation of the saphenous vein, and less invasive methods (13,18).
Patel et al. identified that lymphatic and cutaneous-related are the most common complications in a systematic review and meta-analysis, comprising of eight articles, reported complications (two trials that were randomized and six observational studies) compiling data on almost 500 patients who underwent ILND (213 patients using a minimally invasive surgery (MIS) approach and 283 open (8).
Gopman et al. (19) reported the overall complication rate for open ILND to be 55.4% in a large series. Using the Clavien Dindo classification system (20), 65.7% of those complications were reported as minor. The amount of lymph nodes taken out during a surgery was found to be a factor that can predict the likelihood of postoperative complications (12). Other predictors for complications were Sartorius muscle interposition and increased age (21). However, other authors have reported that body mass index (BMI), diabetes, smoking status, and patients with ≥1 comorbidity are also predictors for perioperative complications as well. Comparative studies between open-ILND and MIS-ILND techniques have demonstrated that MIS-ILND is linked with reduced morbidity, specifically cutaneous and infection-related complications (13,14,22-26). No significant difference between MIS techniques has been found. Given that ILND is not a commonly performed procedure, assessing learning curves is cumbersome; hence, appropriate comparisons between laparoscopic and robotic approaches are sparse in the literature (27,28).
Cutaneous-related complications are mainly infectious or associated with tissue ischemia (0.6–43% of all the complications) (19,29,30). Cellulitis is the most common complication reported in this category, comprising up to 7.9% (VEIL) and 68% (open) of the cutaneous complications reported in the literature, followed by skin necrosis (8). One factor that may impact infectious-related complications is the proper antibiotic usage targeting gram-negative rods and staphylococcus aureus (31). Based on current guidelines, a single dose of cefazolin or ampicillin/sulbactam is recommended (31).
The meticulous creation of the working space for this procedure in which Camper’s space is preserved is critical as well as port placement, and postoperative care to avoid any ischemic cutaneous complications (32). Skin necrosis is found in up to 6% and 30% of VEIL, and open-ILND, respectively (8). Theoretically, MIS approaches should have fewer wound complications by limiting the amount of skin affected during the surgery, less traction, and better cosmesis compared to open (13,14,23,32,33).
The most common vascular complication after ILND is deep vein thrombosis (DVT) (21,34). The risk of developing these types of complications, such as DVT, is increased in patients undergoing ILND due to the nature of the surgery, underlying malignancy, and prolonged postoperative bed rest (21). Most authors still recommend low molecular weight heparin subcutaneously pre-and post-operatively or direct oral anticoagulant agents to decrease the risk of DVT (21,33). It is advised that early mobilization along with the use of sequential compression devices be utilized to help prevent DVT (21,23,32,33). In case compressive garments are used, they can be used for six months postoperatively and should be used from the ankles up to a few centimeters below the umbilicus. Thorough care should be taken in cases where the flap is deemed too thin, as the external compression of the garment can further decrease skin blood flow and cause skin necrosis (23,32).
Seroma and lymphocele are reported as complications in up to 24% and 4%, respectively (21,29). It is suggested that postoperative suction drains be utilized until there is an output of less than 50 mL within 24 hours (21,22,24,26,33), usually around two weeks after the surgical procedure. Some surgeons may get the false perception that early drain removal correlates to good surgical performance. If the collection of serum (seroma) or lymph (lymphocele) in the tissues occurs after early drain removal, the patient may need to go through several needle aspirations to treat the collection, which in the end, predisposes to a greater risk of infectious complications. Prolonged drainage time can lead to an increased risk of infectious complications from the colonization of the drains (33). An important factor is whether the usage of bulb suction or not regarding drain management. In our experience, we believe that prolonged suctioning may prolong and increase the inflammatory reaction in the surgical area, which could delay the natural healing process of the area and the lymphatic channels during the postoperative period.
Lymphedema is the most commonly reported field among lymphatic-related complications (35). The prevalence of limb lymphedema varies among studies depending on the definition used and the duration of follow-up, which remains heterogeneous and not yet universally accepted (23). One proposal to decrease lymphatic complications by improving the lymph circulation of the region was proposed by Catalona et al. (18) with saphenous vein preservation. Following the same principle, a randomized comparison by Zhang et al. (36) reported 32% of limb lymphedema in the saphenous vein-spared group against 70% of lymphedema in the vein-ligated group. These findings are consistent with the findings reported by Yuan et al. (37). Some studies, however, show no difference in the complication rate whether the saphenous vein is preserved or ligated (25). Another hypothesis to explain the different rates of lymphatic leak is the energy mechanism used for the intraoperative management of the lymphatic vessels during surgery. Our experience suggests that ultrasonic devices effectively control most lymphatic vessels during ILND; however, some vessels should be controlled with clips. We recommend clipping every large-sized lymphatic vessel (21,25). Preserving the camper’s layer might be also relevant for the formation of lymphedema, given that you will be also preserving small lymphatics the course through that layer, which might serve as a collateral route for drainage.
Moreover, several studies have proposed the importance of lymph flow imaging techniques with indocyanine green (ICG) or methylene blue to identify lymphatic vessels to reduce the risk of lymphorrhea and lymphatic leaks (38-40). Additionally, collagen or fibrin sealants have also been proposed to seal leaking lymphatic capillaries or fill dead spaces in the surgical area, avoiding the accumulation of fluids in the area, in order to decrease postoperative lymphorrhea. Yet, more data on the clinical utility of these sealant techniques on postoperative complications are needed. Other strategies to minimize lower-extremity lymphedema postoperatively include the same used to decrease the risk of DVT, which encompass the use of elastic stockings and early ambulation (32,33,41).
Nerve/musculoskeletal complications or sequelae (90 days), such as inguinal paresthesia or functional-related adverse events, can be noted. Reports detailing the results of ILND rarely discuss the incidence of complications associated with the procedure (21,42).
Heterogeneity in perioperative complications and adverse events assessment and reporting methods across the literature causes a lack in consistency, various terminologies are used for the same complication, which hinders the ability to standardize the information and conduct precise analyses, resulting in a lack of discussion on the incidence of complications following ILND in many reports. The lack of standardization in reporting complications across studies hinders the ability to compare and analyze data, ultimately impeding the development of effective preventative or management strategies. Furthermore, penile cancer is rarely seen in industrialized nations, making it difficult to undertake expansive prospective randomized studies.
Experienced surgeons, early recognition, and timely complications management are paramount in the management of penile cancer. Though steady advances in surgical techniques and postsurgical care have been made (33), there is still room for improvement to decrease the morbidity of this procedure.
Oncological results and follow-up after surgery
Oncological control
The reported 5-year survival percentage for SCC of the penis without inguinal lymph node metastases ranges from 40% to 100%, with an average survival rate of roughly 75% (43). The 5-year survival rate of individuals with surgically excised lymph node metastases can range from 0 to 80%, with an approximate average of 60% (43). The extent of nodal metastases determines this wide range (43). In men with minimal node metastases (1–2 nodes), the 5-year survival rate ranges from 75% to 90%. However, those with more than 2 involved nodes, extranodal extension of cancer, lymph nodes greater than 4 cm in diameter, or pelvic node metastases have an average survival rate of 5% to 50%. This is much lower than the former, with rates as low as 5% to 10% (44,45).
Studies indicate that ILND may be a curative procedure for up to 60% of those with node-positive diagnosis, though 5-year survival is not guaranteed even for those with node-negative status, with failure rates spanning from 5% to 20% (45).
Follow-up
Despite the evidence that suggests it, some medical professionals are reticent to proceed with an ILND, due to worries surrounding the high rate of difficulties linked to the operation.
According to a recent report, only 50% of the 454 patients registered in Sweden’s National Penile Cancer Register between 2000 and 2003 who had a G2–3 pT1 primary tumor and were considered to be at high risk for inguinal metastases underwent an ILND as suggested by guidelines from the European Association of Urology.
Although selecting patients for ILND based on risk groups may be useful, it is not necessarily a definitive approach and has certain limitations. As per Leijte et al., the incidence of node metastases was only 6% in patients in the low-risk group. However, the incidence of inguinal node metastases was 54% in the intermediate risk group, and 37% in the high risk group, implying that between 46% and 63% of these patients do not have metastases to their inguinal nodes (46).
Studies conducted recently suggest that the overall survival (OS) and 5-year survival between ILND and the VEIL techniques were similar; 88 vs. 80 months (P=0.840) and 65% vs. 66.8% (P=0.636) respectively (47).
Nerveless, the study is limited to a select group of authors who have demonstrated expertise in applying techniques across diverse materials and socioeconomic populations. Nevertheless, the similarity results of their methodologies lend credibility to the article’s presentation of these techniques. A summary of the results of the main series of VEIL are included in Table 1.
Table 1
| Series | Number | Number of lymphade-nectomies | Operative time (min) | Removed lymph nodes | Days to drainage removal | Hospital length of stay (days) | Follow-up (months) |
|---|---|---|---|---|---|---|---|
| Yadav et al. (48) | 29 | 29 | 162.83 | 7.6 (7 to 8) | 2 (2 to 4) | 4.65 (4 to 8) | 14 (7 to 28) |
| Romanelli et al. (49) | 20 | 33 | 119 (55 to 210) | 8 (3 to 16) | (3 to 21) | 5 (2 to 10) | 20 (2 to 36) |
| Kumar et al. (26) | 20 | 33 | 97 | 9.36 | – | 2.5 (0 to 14) | 16 (4 to 35) |
| Wang et al. (22) | 16 | 19 | 139.5±45.52 | 10.78±5.22 | 7.23±1.79 | 10.43±2.53 | – |
| Chaudhari et al. (50) | 14 | 22 | 194.86 (178 to 210) | 7.68 (5 to 11) | – | – | 48 |
| Sotelo et al. (13) | 8 | 14 | 31 (50 to 150) | 9 (4 to 15) | – | – | – |
| Pahwa et al. (51) | 10 | 10 | 144 (120 to 180) | 10.6 (7 to 12) | 5.1 (4 to 8) | – | (3 to 14) |
| Canter et al. (52) | 10 | 19 | 177.5 (132 to 400) | 11 (3 to 26) | 25 (8 to 101) | 1 (1 to 12) | – |
| Tobias-Machado et al. (53) | 10 | 10 | 126 (90 to 130) | 10 (6 to 16) | 4.9 (3 to 8) | – | 18.7 (12 to 31) |
| Tobias-Machado et al. (23) | 15 | 20 | 120 (90 to 160) | 10.75 (6 to 16) | 4.9 (3 to 12) | (0.5 to 10) | 29.05 |
| Subirá-Rios et al. (16) | 10 | 20 | 147 (120 to 170) | 10.25 (8 to 14) | 4.7 (3 to 9) | 5.8 (3 to 10) | 18 |
Data are shown as mean, mean (range) or mean ± standard deviation. VEIL, video endoscopic inguinal lymphadenectomy.
Conclusions
Oncologic Surgeons who deal with that pathology should use the fearsome techniques VEIL, L-VEIL, H-VEIL and R-VEIL should strive to provide the best possible care for patients with both positive and negative lymph nodes. As this procedure becomes more common, studies in the future and now are proving how safe endoscopic surgery is with less morbidity, convalescence and similar oncological characteristics.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Stênio de Cássio Zequi) for the series “Penile Cancer” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-8/coif). The series “Penile Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no personal or business interest in or potential for personal gain from any of the organizations or projects linked to Ligasure™ and scalpel® (Ethicon, CA, USA)/Ligasure® (Covidien, MA, USA) cited in text. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures presented in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Skeppner E, Andersson SO, Johansson JE, et al. Initial symptoms and delay in patients with penile carcinoma. Scand J Urol Nephrol 2012;46:319-25. [Crossref] [PubMed]
- Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol 2010;57:1002-12. [Crossref] [PubMed]
- Pettaway CA, Pisters LL, Dinney CP, et al. Sentinel lymph node dissection for penile carcinoma: the M. D. Anderson Cancer Center experience. J Urol 1995;154:1999-2003. [Crossref] [PubMed]
- Ornellas AA, Seixas AL, Marota A, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J Urol 1994;151:1244-9. [Crossref] [PubMed]
- Hakenberg O, Compérat E, Minhas S, et al. European Association of Urology Guidelines: penile cancer. Uroweb 2019. Available online: https://uroweb.org/guideline/penile-cancer/
- Chen G, Wang Y, Zhu H, et al. Video endoscopic inguinal lymphadenectomy via hypogastric/limb subcutaneous approach for early-stage vulvar cancer. Zhonghua Yi Xue Za Zhi 2014;94:39-42. [PubMed]
- Kandasamy SG, Chandran KR, Pooleri GK. Minimal invasive approaches in lymph node management of carcinoma of penis: A review. Indian J Urol 2022;38:15-21. [Crossref] [PubMed]
- Patel KN, Salunke A, Bakshi G, et al. Robotic-Assisted Video-Endoscopic Inguinal Lymphadenectomy (RAVEIL) and Video-Endoscopic Inguinal Lymphadenectomy (VEIL) versus Open Inguinal Lymph-Node Dissection (OILND) in carcinoma of penis: Comparison of perioperative outcomes, complications and oncological outcomes. A systematic review and meta-analysis. Urol Oncol 2022;40:112.e11-22. [Crossref] [PubMed]
- Nabavizadeh R, Petrinec B, Nabavizadeh B, et al. Inguinal lymph node dissection in the era of minimally invasive surgical technology. Urol Oncol 2023;41:1-14. [Crossref] [PubMed]
- Slaton JW, Morgenstern N, Levy DA, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol 2001;165:1138-42. [Crossref] [PubMed]
- Correa AF, Handorf E, Joshi SS, et al. Differences in Survival Associated with Performance of Lymph Node Dissection in Patients with Invasive Penile Cancer: Results from the National Cancer Database. J Urol 2018;199:1238-44. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Nieweg OE. Contemporary management of penile squamous cell carcinoma. J Surg Oncol 2005;89:43-50. [Crossref] [PubMed]
- Sotelo R, Sánchez-Salas R, Carmona O, et al. Endoscopic lymphadenectomy for penile carcinoma. J Endourol 2007;21:364-7; discussion 367. [Crossref] [PubMed]
- Tobias-Machado M, Tavares A, Molina WR Jr, et al. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int Braz J Urol 2006;32:316-21. [Crossref] [PubMed]
- Riveros M, Garcia R, Cabañas R. Lymphadenography of the dorsal lymphatics of the penis. Technique and results. Cancer 1967;20:2026-31. [Crossref] [PubMed]
- Subirá-Ríos D, Caño-Velasco J, Moncada-Iribarren I, et al. Pelvic and inguinal single-site approach: PISA technique. New minimally invasive technique for lymph node dissection in penile cancer. Actas Urol Esp 2022;46:150-8. (Engl Ed). [PubMed]
- Matin SF, Cormier JN, Ward JF, et al. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 2013;111:1068-74. [Crossref] [PubMed]
- Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J Urol 1988;140:306-10. [Crossref] [PubMed]
- Gopman JM, Djajadiningrat RS, Baumgarten AS, et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int 2015;116:196-201. [Crossref] [PubMed]
- Clavien PA, Barkun J, De Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol 2009;27:205-12. [Crossref] [PubMed]
- Wang S, Du P, Tang X, et al. Comparison of Efficiency of Video Endoscopy and Open Inguinal Lymph Node Dissection. Anticancer Res 2017;37:4623-8. [PubMed]
- Tobias-Machado M, Tavares A, Silva MN, et al. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol 2008;22:1687-91. [Crossref] [PubMed]
- Singh A, Jaipuria J, Goel A, et al. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J Urol 2018;199:1518-25. [Crossref] [PubMed]
- Shao Y, Hu X, Ren S, et al. Comparison of different surgical methods and strategies for inguinal lymph node dissection in patients with penile cancer. Sci Rep 2022;12:2560. [Crossref] [PubMed]
- Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int 2017;119:530-4. [Crossref] [PubMed]
- Russell CM, Salami SS, Niemann A, et al. Minimally Invasive Inguinal Lymphadenectomy in the Management of Penile Carcinoma. Urology 2017;106:113-8. [Crossref] [PubMed]
- Gkegkes ID, Minis EE, Iavazzo C. Robotic-assisted inguinal lymphadenectomy: a systematic review. J Robot Surg 2019;13:1-8. [Crossref] [PubMed]
- Stuiver MM, Djajadiningrat RS, Graafland NM, et al. Early wound complications after inguinal lymphadenectomy in penile cancer: a historical cohort study and risk-factor analysis. Eur Urol 2013;64:486-92. [Crossref] [PubMed]
- Koifman L, Hampl D, Koifman N, et al. Radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes. J Urol 2013;190:2086-92. [Crossref] [PubMed]
- Lightner DJ, Wymer K, Sanchez J, et al. Best Practice Statement on Urologic Procedures and Antimicrobial Prophylaxis. J Urol 2020;203:351-6. [Crossref] [PubMed]
- Sotelo R, Sanchez-Salas R, Clavijo R. Endoscopic inguinal lymph node dissection for penile carcinoma: the developing of a novel technique. World J Urol 2009;27:213-9. [Crossref] [PubMed]
- Sood A, Rudzinski JK, Spiess PE, et al. The Acute Complications After Surgery for Penile Carcinoma and Strategies for Their Management: A Systematic Review of the Literature. Semin Oncol Nurs 2022;38:151285. [Crossref] [PubMed]
- Gupta MK, Patel AP, Master VA. Technical considerations to minimize complications of inguinal lymph node dissection. Transl Androl Urol 2017;6:820-5. [Crossref] [PubMed]
- Witte MH, Bernas MJ. Evolution of the 2020 international society of lymphology consensus document parallels advances in lymphology: an historical perspective. Lymphology 2020;53:1-2. [PubMed]
- Zhang X, Sheng X, Niu J, et al. Sparing of saphenous vein during inguinal lymphadenectomy for vulval malignancies. Gynecol Oncol 2007;105:722-6. [Crossref] [PubMed]
- Yuan P, Zhao C, Liu Z, et al. Comparative Study of Video Endoscopic Inguinal Lymphadenectomy Through a Hypogastric vs Leg Subcutaneous Approach for Penile Cancer. J Endourol 2018;32:66-72. [Crossref] [PubMed]
- Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;127:1979-86. [Crossref] [PubMed]
- Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg 2011;128:314e-21e. [Crossref] [PubMed]
- Ravisankar P, Malik K, Raja A, et al. Clipping inguinal lymphatics decreases lymphorrhoea after lymphadenectomy following cancer treatment: results from a randomized clinical trial. Scand J Urol 2021;55:480-5. [Crossref] [PubMed]
- Rabe E, Partsch H, Hafner J, et al. Indications for medical compression stockings in venous and lymphatic disorders: An evidence-based consensus statement. Phlebology 2018;33:163-84. [Crossref] [PubMed]
- Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. J Urol 2002;167:1638-42. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile sparing surgery for penile cancer-does it affect survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
- Spratt J. Groin dissection. J Surg Oncol 2000;73:243-62. [Crossref] [PubMed]
- Johnson DE, Lo RK. Management of regional lymph nodes in penile carcinoma. Five-year results following therapeutic groin dissections. Urology 1984;24:308-11. [Crossref] [PubMed]
- Leijte JA, Kerst JM, Bais E, et al. Neoadjuvant chemotherapy in advanced penile carcinoma. Eur Urol 2007;52:488-94. [Crossref] [PubMed]
- Thyavihally YB, Dev P, Waigankar SS, et al. Comparative study of perioperative and survival outcomes after video endoscopic inguinal lymphadenectomy (VEIL) and open inguinal lymph node dissection (O-ILND) in the management of inguinal lymph nodes in carcinoma of the penis. J Robot Surg 2021;15:905-14. [Crossref] [PubMed]
- Yadav SS, Tomar V, Bhattar R, et al. Video Endoscopic Inguinal Lymphadenectomy vs Open Inguinal Lymphadenectomy for Carcinoma Penis: Expanding Role and Comparison of Outcomes. Urology 2018;113:79-84. [Crossref] [PubMed]
- Romanelli P, Nishimoto R, Suarez R, et al. Video endoscopic inguinal lymphadenectomy: surgical and oncological results. Actas Urol Esp 2013;37:305-10. [Crossref] [PubMed]
- Chaudhari R, Khant SR, Patel D. Video endoscopic inguinal lymphadenectomy for radical management of inguinal nodes in patients with penile squamous cell carcinoma. Urol Ann 2016;8:281-5. [Crossref] [PubMed]
- Pahwa HS, Misra S, Kumar A, et al. Video Endoscopic Inguinal Lymphadenectomy (VEIL)--a prospective critical perioperative assessment of feasibility and morbidity with points of technique in penile carcinoma. World J Surg Oncol 2013;11:42. [Crossref] [PubMed]
- Canter DJ, Dobbs RW, Jafri SM, et al. Functional, oncologic, and technical outcomes after endoscopic groin dissection for penile carcinoma. Can J Urol 2012;19:6395-400. [PubMed]
- Tobias-Machado M, Tavares A, Ornellas AA, et al. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol 2007;177:953-7; discussion 958. [Crossref] [PubMed]
Cite this article as: Salazar-M-Messias I, Sotelo R, Subirá-Rios D, Elbalka S, Medina LG, Corona-Montes VE, Saba ASS, Hidaka AK, Tobias-Machado M; Penile Cancer Collaborative Coalition Latin-America. Techniques, outcomes, and complications of minimally invasive inguinal lymphadenectomy in cancer of the penis: an international clinical review. AME Med J 2023;8:24.

