Complications following bronchoscopic lung volume reduction (BLVR)
Patients with severe emphysema suffer from chronic dyspnea, poor exercise tolerance, and suboptimal quality of life. Despite typical treatments for emphysema which include pharmacotherapy, smoking cessation, pulmonary rehabilitation and supplemental oxygen, patients with emphysema typically remain functionally limited (1). While lung volume reduction surgery (LVRS) can be considered for individuals suffering from end-stage chronic obstructive pulmonary disease (COPD) with upper lobe predominant disease with low exercise capacity, as demonstrated by the NETT trial, it is associated with significant perioperative morbidity and mortality (2,3). Bronchoscopic lung volume reduction (BLVR) is a less invasive and safe alternative to LVRS for patients with severe emphysema (4).
Patients with significant emphysema with associated hyperinflation, coupled with an emphysematous target lobe without collateral ventilation are ideal candidates for BLVR. Bronchoscopic placement of endobronchial valves (EBVs) induces atelectasis of a target lobe with an associated reduction in residual volume (RV). This reduction results in improved diaphragmatic movement, with a decreased dependence on intercostal and accessory muscles.
Post-procedural complications following BLVR include pneumothorax, COPD exacerbations, and valve dislodgement. These complications typically occur in the immediate post-procedural period, within 72 hours of the procedure. Close monitoring of patients following BLVR is essential because of the underlying comorbidities of the patient population. After valve placement, if patients develop persistent cough, recurrent pneumonia, hemoptysis, or chest discomfort, computed tomography (CT) scans and bronchoscopy are typically offered to assess for complications (5).
The development of a unilateral simple pneumothorax is the most common complication associated with BLVR (6). In rarer cases, patients may develop post obstructive pneumonia, hemoptysis, airway kinking, shunting related hypoxia, and persistent cough related to granulation tissue, valve migration, malposition, and airway tenting as reported by the LIVE, BeLieVeR-HiFi, and TRANSFORM trials (7-9).
Post-procedure pneumothorax is more likely to develop in patients who develop rapid atelectasis (Figure 1). The consideration of pneumothorax as a “complication” is somewhat debatable as this result may be more of a treatment effect. Conformational change in the collapsed lung after valve placement may lead to tears in ipsilateral, untreated lobe of the lung, leading to a pneumothorax. Another mechanism of pneumothorax in these patients may be the sudden change in elastic recoil after valve placement with alterations in the tension in bullous areas of lung.
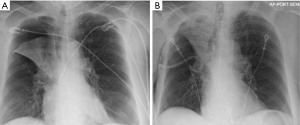
Intercostal drainage using a chest-tube is the standard treatment for a simple unilateral pneumothorax following BLVR. A variety of treatment options can be considered for more complex cases with a high-flow air leak. In most cases, standard management of a persistent high-flow air leak includes observing the patient in the hospital until resolution or discharging home with a chest tube. In some cases, especially when a patient cannot be liberated from wall-suction or if significant subcutaneous emphysema develops, either temporary removal of a single valve, or complete removal of all valves should be considered. A prospective study by Herzog et al. investigated if differences in post-procedure care influenced rates of pneumothorax development (10). They showed that patients who underwent bedrest for 48 hours with cough suppressant therapy had significantly lower rates of pneumothorax, from 25% to 5%. Identifying those at higher risk for development of pneumothorax may help guide decisions regarding which patients need closer monitoring. Gompelmann et al. showed that patients with low attenuation volume of the ipsilateral untreated lobe, low ipsilateral untreated lobe volume/hemi thorax ratio, emphysema type, pleural adhesions, and low RV were predictors for development of pneumothorax (11).
Pneumothorax ex vacuo is an unfilled space within the pleural cavity. This results from the inability of the untreated, ipsilateral lobe to expand into the vacant pleural cavity after target lobe deflation. Pneumothorax ex vacuo typically does not require intercostal thoracotomy drainage and is typically self-resolving (12).
The LIBERATE trial was the pivotal multicenter randomized controlled trial that assessed the effectiveness and safety of Zephyr EBVs and showed improvements in lung function, exercise tolerance, dyspnea, and improved quality out life after 12 months (13). Simple, unilateral pneumothorax was the most common complication after EBV placement at 27% frequency, with 76% of pneumothoraces occurring within 3 days after index procedure.
Acute COPD exacerbation is the second most common complication following EBV placement, with a rate ranging anywhere from 4.6% to 42.3% (14). This wide range is thought to be related to subjective clinical diagnosis of a COPD exacerbation, varying practices among centers, as well as variations in post-procedure time frames. Abia-Trujillo et al. conducted a multicenter retrospective analysis to assess the effects of prophylactic steroids and antibiotics on COPD exacerbations. They defined acute COPD exacerbation as a sustained acute worsening of a patient’s respiratory symptoms beyond normal day-to-day variations leading to a medication change, as stated by the Global Initiative for Chronic Obstructive Lung Disease (GOLD). Worsening symptoms were considered increased cough, sputum production or dyspnea. The period of “acute” was defined as onset of symptoms occurring within first 90 days after EBV placement. They showed that patients who received prophylaxis had significantly lower rates of acute COPD exacerbation than those who did not (16.7% vs. 46.2%, P=0.0001). The rate was lowest in the group who received antibiotics alone vs. those who received both, or steroids alone (15).
The presence of adhesions increases the chances of complications following BLVR. Adhesions are reported in up to 18% of patients undergoing EBV placement. Central airway tenting is seen after valve induced atelectasis as the position of the remainder lobes and airways changes. Occasionally, this results in bronchial folding and airway narrowing and bronchial angulation. This phenomenon has been reported in less than 5% of patients undergoing the procedure (5). Patients with bronchial angulation may present with persistent cough, dyspnea, and mucus plugging. If symptoms are mild, valves do not necessarily have to be removed. Other reasons for poor valve function are related to excessive mucus impaction, or bacterial or fungal colonization. Rarer infectious complications include fungal infections of the valve, lobar abscesses and cavitary lesions. There is limited literature regarding direct fungal infections of EBVs, though those who are colonized with candida albicans are at higher risk (16). Along with antifungal therapy, these valves often require removal for source control. Similarly, those with cavitary lesions should be worked up for nontuberculous mycobacteria infections and mycobacterium tuberculosis, particularly for those who are immunocompromised. These patients require prolonged courses of treatment, and sometimes require valve removal.
Dislodgement of an EBV may lead to partial airway obstruction and allowing for expansion of the target lobe (17). Valve dislocation can occur due to granulation tissue formation, bronchomalacia, or bronchitis. Valve migration can occur spontaneously, when there is mispositioning, or when an incorrect EBV size is placed. Clinically, valve migration can be detected with sudden dyspnea and chest discomfort. When CT imaging suggests valve dislocation or untreated airway, patients need to undergo revision bronchoscopy. Even if CT imaging is inconclusive, a revision bronchoscopy is sometimes necessary to assess for small missed subsegments or to assess partially misplaced valves (18).
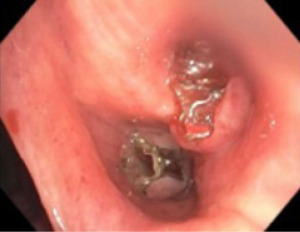
Minor hemoptysis can also occur following BLVR procedure and is often self-limited. Most cases of hemoptysis are due to granulation tissue formation at the site of the valves or mucosal ulceration due to valve movement (Figure 2). If the degree of hemoptysis is significant, bronchoscopic evaluation is necessary. If hemoptysis is severe, valve removal or even endovascular interventions may be necessary.
There are important factors to consider when assessing the success of EBV placement, the most important being the absence of collateral ventilation between the treatment target and ipsilateral lobe, which is primarily verified using the Chartis System. Despite ruling out the presence of collateral ventilation, responder rates varied widely in four large clinical trials (IMPACT, STELVIO, TRANSFORM, LIBERATE) that used Chartis, with responder rates ranging anywhere from 40% to 87%. Still, only 13% of these patients underwent permanent EBV removal. One hypothesis that has gained traction is that distribution of emphysema plays a larger than previously realized role in success outcome. Hartman et al. showed in a cohort of 428 patients with a mean 38 pack year smoking history, that non-responders had significantly less destruction, less air trapping, and higher perfusion in the target lobe than responders. Given their findings, they concluded that those with a more homogenous distribution of emphysema (with less air trapping and more perfusion) were less likely to be non-responders to EBV placement (19).
Understanding the concept of air trapping and how this concept relates to successful EBV placement is important. Over time, air trapping and lung hyperinflation develop in patients with COPD, with eventual obstruction with expiratory flow limitations, and both static and dynamic hyperinflation. Conventionally, hyperinflation has been defined by a total lung capacity (TLC) of >120% of the predicted value. Others suggest that RV or RV/TLC may be more reliable for assessing static hyperinflation. Other studies suggest that plethysmography derived TLC can overestimate volume, especially in severe obstruction, whereas helium may underestimate TLC due to difficulty for the gas to reach parts of the lung. These considerations are important when assessing optimal target lobes for EBV placement as the degree of air trapping and heterogeneity of the diseased lobe may differ depending on the method used for assessment. These disparities may contribute to some of the inconsistent conclusions that have been made by different studies (IMPACT, STELVIO, TRANSFORM, LIBERATE) regarding outcome success between patients with homogeneous and heterogeneous disease (20).
BLVR offers benefits in terms of lung function and quality of life that is safe for patients with advanced emphysema Though there are potential risks and adverse effects to consider, benefits typically outweigh risks. As EBV technology continues to evolve these risks may become less significant. Ineffective results after EBV placement remains one of the most undesirable outcomes, and is typically due to the presence of collateral ventilation, mucus impaction, and/or bronchial hyper responsiveness after valve placement. Optimal patient selection with regards to not just the absence of collateral ventilation, but also with regards to heterogeneity of the target lobe, degree of air trapping (and how air trapping is measured), perfusion, among other factors, need to be considered in a holistic multidisciplinary fashion moving forward to increase the likelihood of a successful outcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-106/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-106/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. AKM received consulting fees from Pulmonx. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Safka KA, McIvor RA. Non-pharmacological management of chronic obstructive pulmonary disease. Ulster Med J 2015;84:13-21. [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT): Part I: Lessons learned about emphysema. Am J Respir Crit Care Med 2011;184:763-70. [Crossref] [PubMed]
- Criner GJ, Cordova F, Sternberg AL, et al. The National Emphysema Treatment Trial (NETT) Part II: Lessons learned about lung volume reduction surgery. Am J Respir Crit Care Med 2011;184:881-93. [Crossref] [PubMed]
- Ingenito EP, Wood DE, Utz JP. Bronchoscopic lung volume reduction in severe emphysema. Proc Am Thorac Soc 2008;5:454-60. [Crossref] [PubMed]
- Klooster K, Slebos DJ. Endobronchial Valves for the Treatment of Advanced Emphysema. Chest 2021;159:1833-42. [Crossref] [PubMed]
- Fiorelli A, D'Andrilli A, Bezzi M, et al. Complications related to endoscopic lung volume reduction for emphysema with endobronchial valves: results of a multicenter study. J Thorac Dis 2018;10:S3315-25. [Crossref] [PubMed]
- Skowasch D, Fertl A, Schwick B, et al. A Long-Term Follow-Up Investigation of Endobronchial Valves in Emphysema (the LIVE Study): Study Protocol and Six-Month Interim Analysis Results of a Prospective Five-Year Observational Study. Respiration 2016;92:118-26. [Crossref] [PubMed]
- Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (TRANSFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Herzog D, Poellinger A, Doellinger F, et al. Modifying Post-Operative Medical Care after EBV Implant May Reduce Pneumothorax Incidence. PLoS One 2015;10:e0128097. [Crossref] [PubMed]
- Gompelmann D, Lim HJ, Eberhardt R, et al. Predictors of pneumothorax following endoscopic valve therapy in patients with severe emphysema. Int J Chron Obstruct Pulmon Dis 2016;11:1767-73. [Crossref] [PubMed]
- Williams JG, Lerner AD. Managing complications of pleural procedures. J Thorac Dis 2021;13:5242-50. [Crossref] [PubMed]
- Criner GJ, Sue R, Wright S, et al. A Multicenter Randomized Controlled Trial of Zephyr Endobronchial Valve Treatment in Heterogeneous Emphysema (LIBERATE). Am J Respir Crit Care Med 2018;198:1151-64. [Crossref] [PubMed]
- Fitzmaurice GJ, Lau K, Redmond KC. The LIBERATE Trial: Options to Reduce the Risk of Post-procedural Pneumothorax and Length of Stay. Am J Respir Crit Care Med 2018;198:1586-7. [Crossref] [PubMed]
- Abia-Trujillo D, Yu Lee-Mateus A, Garcia-Saucedo JC, et al. Prevention of acute exacerbation of chronic obstructive pulmonary disease after bronchoscopic lung volume reduction with endobronchial valves. Clin Respir J 2022;16:43-8. [Crossref] [PubMed]
- Kim T, Jeon Y, Choi H, et al. Endobronchial fungal infection caused by Candida albicans with main bronchus obstruction: a case report. Oxf Med Case Reports 2019;2019:omz055. [Crossref] [PubMed]
- Valipour A. Valve therapy in patients with emphysematous type of chronic obstructive pulmonary disease (COPD): from randomized trials to patient selection in clinical practice. J Thorac Dis 2018;10:S2780-96. [Crossref] [PubMed]
- Roodenburg SA, Klooster K, Hartman JE, et al. Revision Bronchoscopy After Endobronchial Valve Treatment for Emphysema: Indications, Findings and Outcomes. Int J Chron Obstruct Pulmon Dis 2021;16:1127-36. [Crossref] [PubMed]
- Hartman JE, Roodenburg SA, van Dijk M, et al. Response to endobronchial valve treatment: it's all about the target lobe. ERJ Open Res 2023; [Crossref] [PubMed]
- D'Ascanio M, Viccaro F, Calabrò N, et al. Assessing Static Lung Hyperinflation by Whole-Body Plethysmography, Helium Dilution, and Impulse Oscillometry System (IOS) in Patients with COPD. Int J Chron Obstruct Pulmon Dis 2020;15:2583-9. [Crossref] [PubMed]
Cite this article as: Chakravorty S, Mahajan AK. Complications following bronchoscopic lung volume reduction (BLVR). AME Med J 2023;8:31.