Bronchoscopic treatment of thoracic malignancy
Introduction
Background
Thoracic oncology is a rapidly evolving field that focuses on studying and treating cancers in the chest region. Within this field, interventional pulmonologists face significant challenges in managing malignant central airway obstruction (MCAO) and peripheral lung malignancies.
Rationale and knowledge gap
The bronchoscopic management of MCAO and peripheral lung malignancies presents specific areas that require special attention and exploration. Numerous therapeutic bronchoscopic modalities, encompassing ablative procedures and the use of airway stents, are available. Nevertheless, the strategic application of these modalities, either singly or in combination, has been sparingly addressed in prior literature. While the utilization of these modalities has been individually examined in distinct scenarios, such as early-stage lung cancer and peripheral lung malignancies, there is a notable scarcity of comparative analyses between them.
Objective
Our objective in this review is to explore and discuss the bronchoscopic management of MCAO and peripheral lung malignancies, aiming to contribute to improved patient care strategies in thoracic oncology.
MCAO
MCAO is characterized by a luminal obstruction of over 50% of the trachea, mainstem bronchi, bronchus intermedius or a lobar bronchus (1). Patients with MCAO may experience symptoms such as cough, hemoptysis, and shortness of breath, which can range from mild to severe respiratory distress (2). The diagnosis of MCAO is often made through CT imaging of the chest, which is both sensitive and non-invasive (3). MCAO is known to be associated with a decrease in quality of life and an increase in mortality (4).
MCAO can have various causes, with lung cancer being the most common. Up to 30% of patients with lung cancer develop clinically evident MCAO during the course of their disease (2). Other malignancies that more commonly cause MCAO include tumors of airway origin such as adenoid cystic carcinoma (ACC), carcinoid tumor, and mucoepidermoid carcinoma. Intrathoracic extrapulmonary malignancies, such as esophageal, thyroid cancer, and lymphoma, as well as extrathoracic metastases, such as head and neck cancer, renal cell carcinoma, and colon cancer, are all known to spread directly to the airways (2,5,6).
Regardless of the underlying cause, MCAO can be classified into three major subtypes: intrinsic, extrinsic, and mixed obstruction. Intrinsic or endoluminal obstruction refers to a tumor that is confined inside the airway lumen. Extrinsic or extraluminal obstruction occurs when mass outside the airway within the adjacent parenchyma compresses and narrows the airway. Mixed obstruction describes a process that involves both intrinsic and extrinsic compression. The Cotton-Myer system can be used to grade MCAO, with Grade I indicating an obstruction of 0–50%, Grade II indicating 51–70%, grade III indicating 71–99%, and grade IV indicating complete obstruction at 100% (7).
Bronchoscopic treatment is typically considered a palliative measure for relieving MCAO (8). However, the immediate success rate of the procedure, defined as more than 50% of luminal patency, is generally high (1). Small retrospective studies have also shown a survival benefit and improvement in quality of life, though this effect is likely more significant in patients with more favorable pre-procedural functional status (9,10).
Anesthesia and airway management
Patients undergoing therapeutic bronchoscopy for MCAO can pose challenges in terms of anesthesia and airway management. In certain patients, concerns arise regarding the anesthetic agents and muscle relaxants that may reduce the muscle tone in the rib cage and diaphragm, leading to loss of airway distending pressure (11). Therefore, adequate ventilation under general anesthesia can be problematic with standard ventilation. In severe cases, the proceduralist needs to consider the risk of catastrophic complete airway obstruction that can occur with full induction of general anesthesia. This may be most relevant in patients with pedunculated mass, or high body mass index (12). In such cases, initial awake flexible bronchoscopy can be advantageous as it permits an airway exam while the patient is spontaneous breathing. It is particularly useful when the clinician is uncertain about the patient’s ability to tolerate increased levels of sedation or the degree of airway obstruction. However, it is important to note that the scope partially occludes about 10–15% of the normal trachea, potentially resulting in a decrease in partial pressure of oxygen (PaO2) by 10–20 mmHg (13).
Clinical practice regarding the initial airway approach for patients with MCAO varies across different centers. Some physicians prefer using a laryngeal mask airway (LMA) or an endotracheal (ET) tube for initial evaluation, transitioning to a rigid bronchoscope if necessary. On the other hand, others opt for direct use of a rigid bronchoscope. No studies have directly compared these two approaches to determine which is superior. Therefore, the choice of approach remains at the discretion of the physician. Compared with LMA and ET tube, rigid bronchoscopy and jet ventilation allow an unobstructed view of the lesion while providing high-pressure air flow that passes the obstruction more easily (14,15). It also offers advantages such as the capability to remove large pieces of tumor, and control bleeding through tamponade, while allowing for the use of multiple instruments (16,17). Barotrauma and hypercapnia can occur with prolonged use (14,15). During airway stenting, employing controlled ventilation with muscle relaxants was found to lower the occurrence of desaturation events and maintain a favorable respiratory status (18). In severe cases, the procedure may require extracorporeal membrane oxygenation (ECMO) support depending on the severity of the obstruction and patient’s comorbidities (19).
Under special circumstances, case reports have suggested the use of non-invasive ventilation (NIV) during therapeutic bronchoscopy in patient with MCAO (20-22). However, the precise role of NIV in this context remains unclear, as it is not considered a stable airway management technique.
Ablative modalities
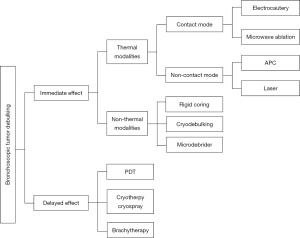
Ablative therapies are commonly employed to reduce tumor size in the airway. These therapies can be categorized into those that have an immediate effect and those with a delayed effect. Among the ablative modalities with immediate effect, two main types can be distinguished: thermal and non-thermal methods (Figure 1). Thermal ablative modalities can be further divided into contact and non-contact techniques. Contact thermal ablative modalities typically include electrocautery and microwave ablation (MWA), while non-contact thermal modalities encompass argon plasma coagulation (APC) and laser treatments. On the other hand, non-thermal ablative modalities that result in immediate tumor debulking include rigid coring, cryo-debulking and microdebrider techniques. Furthermore, there are several ablative modalities with a delayed effect, such as cryotherapy, cryospray, photodynamic therapy (PDT) and brachytherapy (Table 1).
Table 1
| Modalities | Characteristics |
|---|---|
| Modalities with immediate effect | |
| Thermal modalities | |
| Contact modes | |
| Electrocautery | |
| Mechanism | Converting high-frequency electrical currents to heat energy for tissue coagulation or dissection of tumor tissue (23) |
| Tissue penetration | 1–2 mm (24) |
| Hemostasis/coagulation | Yes |
| Settings | Probes 20 W; snares 30 W; knives 10 W; <5 s per pulse (25) |
| Delivery | Tools used through the working channel of the flexible bronchoscope and placed directly on targeted tissue |
| Advantages | Cost friendly and easy to use (26) |
| Disadvantages | Limited by anatomical locations; require repeated cleaning |
| Fire risk | Require FiO2 <40% |
| MWA | |
| Mechanism | Heating tissue by generating an oscillating electromagnetic field around an ablation device; inducing rotation of polar molecules, such as H2O to create friction and heat; causing cellular death via coagulation necrosis (27) |
| Tissue penetration | Depending on the duration of application |
| Hemostasis/coagulation | Yes |
| Settings | Temperature: 80–90 ℃; duration of application based on visible change of the tissue (28) |
| Delivery | Passing the microwave probe through the working channel of a flexible bronchoscope; the tip of the antennae placed into a visible part of the tumor |
| Advantages | Suitable for ablation around larger vascular structures (29) |
| Disadvantages | Limited clinical evidence |
| Fire risk | Not increased |
| Non-contact modes | |
| APC | |
| Mechanism | Forming an ionized monopolar current by combining argon gas with a high-voltage electrical field (30) |
| Tissue penetration | 2–3 mm (31) |
| Hemostasis/coagulation | Yes, highly effective |
| Settings | Gas flow: 0.3–0.8 LPM (24); power: forced mode 30 W, pulsed mode 10 W |
| Delivery | Passing the probe through the working channel of a flexible bronchoscope; 3 types of probes: straight, side and circumferential fire; tip of the probe placed a few millimeters away from the targeted tissue |
| Advantages | Suitable for treating airways with acute angles |
| Disadvantages | Not ideal for rapid tumor debulking |
| Fire risk | Require FiO2 <40% |
| Laser | Nd:YAG, Nd:YAP, Ho:YAG, and potassium titanyl phosphate (KTP) (32) |
| Mechanism | Different types of lasers have different effects on coagulation and tissue vaporization due to their different wavelengths, which lead to various tissue absorption rates (24) |
| Tissue penetration | Up to 10 mm for Nd:YAG (33) |
| Hemostasis/coagulation | Yes, highly effective |
| Settings | For Nd:YAG: power 20–40 W; pulse duration 0.5–1 s |
| Delivery | Passing the fiber through the working channel of a flexible bronchoscope; for Nd:YAG: devascularization: tip to target 10 mm; carbonization or vaporization: tip to target 3–4 mm |
| Advantages | Efficient for debulking and coagulation |
| Disadvantages | Can be challenging to apply to locations with acute angulations; risk of perforation and fistula (24,34); high cost; more training required (35) |
| Fire risk | Require FiO2 <40% |
| Non-thermal modalities | |
| Rigid coring | |
| Mechanism | Mechanically removing tumor using rigid bronchoscope bevel |
| Tissue penetration | – |
| Hemostasis/coagulation | No |
| Settings | – |
| Delivery | A rigid scope is advanced to the proximal end of the tumor, and the bevel is used as a cutting tool while moving forward and turning left and right |
| Advantages | Effective |
| Disadvantages | Bleeding risk |
| Fire risk | Not increased |
| Cryodebulking | |
| Mechanism | Rapid gas decompression to extrude cryogenic liquefied gas through a probe, resulting in significant drop in surrounding temperature (36) |
| Tissue penetration | Depending on the duration of application |
| Hemostasis/coagulation | No |
| Settings | Universal setting; using foot paddle to control the duration of application; 3 sizes of disposable cryoprobes: 1.1, 1.7, and 2.4 mm |
| Delivery | Passing the probe through the working channel of a flexible bronchoscope; freezing for 3–20 s before rapidly withdrawing from the airway, bringing frozen tumor tissue adherent to the probe (37) |
| Advantages | Effective; maintaining the pathological structure (37) |
| Disadvantages | Bleeding risk; loss of visualization as the entire bronchoscope is withdrawn from the airway while waiting for the specimen to thaw |
| Fire risk | Not increased |
| Microdebrider | |
| Mechanism | A hollow metal tube with a rotating blade that is coupled with suction. Mechanical removal of tumor tissue |
| Tissue penetration | – |
| Hemostasis/coagulation | No |
| Settings | Console speed range: 1,000–3,000 rpm |
| Delivery | A rigid device with both angled and straight tips; can only be used through a rigid bronchoscope |
| Advantages | Rapid debulking and clearance while maintaining direct visualization |
| Disadvantages | Bleeding risk; can only access the trachea and proximal end of the main bronchi |
| Fire risk | Not increased |
| Modalities with delayed effect | |
| Cryotherapy | |
| Mechanism | Low temperature induced cellular crystallization and dehydration, resulting in delayed effects of apoptosis and microthrombi formation |
| Tissue penetration | 2–3 mm |
| Hemostasis/coagulation | Yes |
| Settings | See cryodebulking |
| Delivery | Passing the probe through the working channel of a flexible bronchoscope; placing the probe at the tumor tissue; 3 to 5 repeated 30- to 60-s freeze-thaw cycles at one location |
| Advantages | Low perforation risk, safe to use in close proximity to stents (38) |
| Disadvantages | Limited depth of penetration; not suitable for large tumors or symptomatic MCAO (2,24) |
| Fire risk | Not increased |
| Cryospray | |
| Mechanism | Causing intracellular crystallization; preventing extracellular crystal formation and preserving the matrix (37) |
| Tissue penetration | 2 mm for normal flow; 1 mm for low flow |
| Hemostasis/coagulation | Yes |
| Settings | Normal flow: 25 W, energy 500 J; low flow 12.5 W, energy 250 J; dosimetry 20 s |
| Delivery | Passing the probe through the working channel of a flexible bronchoscope; applying liquid nitrogen directly onto the target tissue |
| Advantages | Maintaining the pathological structure |
| Disadvantages | Barotrauma, pneumothorax (39); arrhythmia, hypoxia, hypotension, perioperative mortality (40) |
| Fire risk | Not increased |
| PDT | |
| Mechanism | Activating a photosensitizer in the target tissue with light (41,42); direct cell death induced by singlet oxygen, vascular damage around the tumor and an indirect immune response (43) |
| Tissue penetration | 5–10 mm (44) |
| Hemostasis/coagulation | Yes |
| Settings | Energy density typically ranges 100–200 J/cm2; both rigid and flexible fibers available in different lengths from 10 to 50 mm |
| Delivery | Porfimer sodium, or Photofrin (Pinnacle Biologics Inc., Bannockburn, IL) given at a dose of 2 mg/kg; passing the fiber through the working channel of a flexible bronchoscope; fiber placing above the lesion |
| Advantages | Effective; low procedure risk |
| Disadvantages | Not an ideal modality for patients who require immediate relief of airway obstruction; photosensitivity (45); high cost (46) |
| Fire risk | Not increased |
| Brachytherapy | |
| Mechanism | The emitted gamma radiation causes DNA breakdown, leading to apoptosis and decreased cell proliferation (47) |
| Tissue penetration | Depending on dosage |
| Hemostasis/coagulation | No |
| Settings | HDR delivers >12 Gy/h, with the dose per session (fraction) varying from ~300–1,000 cGy, which is calculated at 10mm from the source axis. IDR range ~2–10 Gy/h. LDR brachytherapy delivers <2 Gy/h. The treatment dose is prescribed by the radiation oncologist (47) |
| Delivery | Bronchoscopic deployment of radioactive seeds (i.e., iridium-192) within or alongside an endobronchial tumor with the assistance of radiopaque catheter (2,46) |
| Advantages | Effective |
| Disadvantages | Hemorrhage/hemoptysis, radiation bronchitis, airway stenosis, and fistula formation, etc. (48) |
| Fire risk | Not increased |
MCAO, malignant central airway obstruction; FiO2, fraction of inspired oxygen; MWA, microwave ablation; APC, argon plasma coagulation; LPM, liter per minute; Nd:YAG, neodymium-doped yttrium aluminum garnet; Nd:YAP, neodymium-doped yttrium aluminum perovskite; Ho:YAG, Holmium-doped YAG; KTP, potassium titanyl phosphate; HDR, high does rate; LDR, low does rate; IDR, intermediate dose rate.
Airway stents
Airway stents are indicated for patients with pure extrinsic compression, mixed obstruction where significant obstruction remains after tumor debulking, or tracheoesophageal fistula (49,50). They are considered palliative treatment for symptomatic patients with MCAO who experience shortness of breath or post-obstructive pneumonia (51). Stent placement can maintain the benefit of therapeutic bronchoscopy for dyspnea, obstruction recurrence and the need for repeat bronchoscopy (52). The approach to airway stent placement demonstrates global variability, encompassing differences in airway selection, anesthesia methods, and the application of rigid bronchoscopy and fluoroscopy (53,54).
There are two major types of stents available: (I) metallic stents and (II) silicone stents. Metallic stents can be further divided into fixed-diameter stents and self-expanding metallic stents (SEMSs). Metallic stents come in three forms—uncovered, partially covered and covered. Most fixed-diameter stents and SEMSs can be easily placed using prepackaged deployment catheters through existing airways, such as LMA or ET (55,56). It does not always require rigid bronchoscopy for placement. The deployment catheter is placed along the side of the flexible bronchoscopy, and the scope is placed at the proximal end of the stent. Smaller stent deployment catheters can fit through the 2.8 mm working channel of a therapeutic bronchoscope. Both flexible bronchoscope and fluoroscopy can both be used as real-time guidance during SEMS placement. Metallic stents can also be placed under fluoroscopy without bronchoscope visualization (57). Based on the findings of a survey study, 23% of providers from around the world consistently utilized fluoroscopic guidance during tracheobronchial stent placement; while 26.5% employing it selectively for specific cases (54).
Silicone stents come in different shapes, forms and sizes (49). There are straight, hourglass, or Y-shape silicone stents available. Pre-made silicone stents can be customized by cutting them short or creating additional openings. Custom-designed silicone Y stents are also available (58). The selection of a stent hinges upon factors such as the nature of the stenosis, the expertise of the operator, and the range of available equipment (59). The placement of silicone stents always requires rigid bronchoscopy and a stent deployer.
Complications arising from the use of stents in airway management encompass a range of issues, including stent migration/malposition, granulation tissue formation, tumor ingrowth, mucoid impaction, infection, stent fracture, and halitosis (49). It is important to note that the specific complication profiles may vary slightly between metallic stents and silicone stents. For instance, certain studies have indicated a higher risk of granulation tissue with metallic stents (60,61), whereas silicone stents are more prone to migration (62,63). Additionally, the use of silicone stents has been associated with an increased risk of mucus plugging (62). Due to epithelialization, bare metal stents tend to have infrequent migration, however, their removal can be problematic (64). In contrast, covered SEMSs and silicone stents are typically easier to remove. For optimal respiratory outcomes and to mitigate airway complications (65), it is recommended to promptly remove all airway stents.
Combined modalities
All of the aforementioned modalities have demonstrated effectiveness and are frequently utilized in together in clinical practice (1,23). However, previous studies have not identified any modality as superior to the others. Each modality possesses distinct characteristics and indications for application. Decisions on which modalities to use must be decided based on user experience, local resources, as well as specific patient characteristics.
For example, a patient’s fraction of inspired oxygen (FiO2) requirement during bronchoscopy can often limit which ablative modalities can be utilized. Therapies like electrocautery, APC and laser carry a risk of airway fire. Consequently, it is crucial to maintain FiO2 below 40% during application of these techniques (24). For patients who are unable to tolerate a lower FiO2, alternative modalities such as cryodebulking, must be utilized.
Additional, tumor characteristics also dictate which tools may be effect. APC has limited tissue penetration and may not be the most effective method for tumor debulking (31). Nonetheless, it proves effective in achieving hemostasis (24). To achieve immediate tumor debulking, effective modalities include rigid coring, cryodebulking, microdebrider, MVA and certain electrocautery tools, such as snares (refer to Table 1 for details). Both electrocautery and APC are particularly useful for achieving hemostasis (24). In a study by Lee et al., rigid coring was employed for tumor tissue removal, followed by APC application for hemostasis (66). Laser therapy is effective in both debulking and hemostasis, but requires specialized training and is associated with higher equipment cost (35). Cryospray, cryotherapy, PDT and brachytherapy exhibit delayed effects and are not optimal for managing symptomatic high-grade obstruction which immediate stenosis relief is required.
Airway stents play a crucial role in maintaining airway patency and are typically indicated when there is >50% stenosis due to extrinsic or mixed obstruction or when addressing defects like fistulas. According to a randomized control trial, patients who underwent stent placement experienced longer-lasting improvement in dyspnea and fewer unattended bronchoscopies compared to those without stenting, although no significant change in the survival curve was observed (52). It is worth noting that airway stents carry their own complication profiles (49).
In summary, it is a common practice to employ a combination of modalities based on tumor characteristics and the availability of equipment.
Bronchoscopic management in early-stage, airway-limited thoracic malignancy
There are several types of malignancies arising predominantly from the central airways, including squamous cell carcinomas (SCCs), and ACCs, which are the most common malignant tracheal tumors (67-69). Other less common types include carcinoid tumors, mucoepidermoid carcinoma, non-squamous cell bronchogenic carcinomas, sarcomas, and pleomorphic adenomas (70). The adjunctive use of narrow-band imaging, autofluorescence and confocal laser endomicroscopy alongside traditional bronchoscopy has in the identification of malignant lesions within the airway has been reported in the literature though their actual utility is unclear (71-74). Radial probe endobronchial ultrasonography (RP-EBUS) can be used to evaluate the depth of tumor invasion, which is essential in determining the durable efficacy of bronchoscopic management of these lesions. If tumor invasion is seen on RP-EBUS beyond or within the cartilaginous layer, the goal of bronchoscopic ablation of these tumors should be palliative rather than curative (75,76).
There are several bronchoscopic options available, including PDT, cryotherapy, electrocautery, APC and laser. Compared with surgery, endobronchial treatment is less invasive and has a lower morbidity and mortality, with the major risk of potential underestimation of the tumor’s extensiveness (77). The best candidates for bronchoscopic treatments are patients with lesions that are smaller than 10 mm without cartilaginous invasion (78).
PDT is a treatment option for early, airway limited, lung cancer and typically achieves a complete response rate exceeding 70% of cases (43,44,79-84). The success of the treatment is impacted by the size of the lesion, with lesions of SCC smaller than 10 mm having a complete response rate of more than 90%; while those larger than 20 mm have a complete response rate of only 30–40% (44,83). PDT can also be used as the initial treatment to enable less extensive surgical resection (85).
Cryotherapy is another treatment option that has demonstrated a high remission rate of 91% at 1 year in a multicenter study involving 35 patients with bronchogenic carcinoma (86). Cryotherapy was also found to be effective in treating both typical and atypical endoluminal-restricted carcinoid cases (87-90). However, the narrowness of the treatment area and the 3 mm depth of its cytotoxic action are the major pitfalls associated with cryotherapy (91).
Electrocautery is a less commonly used treatment option, but it has shown to have an 80% response rate and no recurrence after 22 months in a small retrospective study (92).
Nd-YAG laser treatment for airway-limited lung cancer has not been extensively investigated. However, a retrospective study demonstrated a complete response rate of 100% among 19 patients with carcinoma in situ (93). The median interval for repeat bronchoscopy was reported to be 100 days, but long-term outcomes were not reported.
Brachytherapy has been studied in a large cohort of 226 patients, which showed a 93.6% compete response rate in 3 months and a 5-year survival of 29% (94). However, fatal hemoptysis occurred in 5% of cases.
Overall, there are several bronchoscopic treatment options available for central airway malignancies. PDT has the largest evidence base for curative intent in early-stage airway malignancies.
Emerging options for MCAO
The field of bronchoscopic management of MCAO is seeing several developments. A robotic system for rigid bronchoscopy was designed to treat CAO, as described in Gafford et al.’s 2020 study (95). The system has two robot arms that pass through a 10 mm rigid bronchoscope. Each arm consists of two pre-curved Nitinol tubes with two degrees of freedom each, allowing for telescopic extension and axial rotation. A monopolar electrosurgical probe was inserted through one of the arms, while a second robotic manipulator was used for retraction and tissue manipulation. However, this system has only been tested on cadavers so far.
In addition, intratumoral injection of chemotherapy has been studied as a treatment for CAO. Mehta et al.’s study found that cisplatin has sustained effects on airway patency when given one to four times, weekly apart (96). Guan et al.’s study showed that para-toluenesulfonamide had a rapid debulking effect when given for 2–3 sessions weekly within 2 weeks to patients with ACC (97). Moreover, a retrospective study of 206 patients found that cisplatin plus rh-endostatin intratumoral injection is effective and safe for the therapy of MCAO caused by primary squamous cell lung cancer (98).
Drug-eluding silicone stents were also studied for the use in lung cancer. The study found that paclitaxel incorporated into a silicone matrix in airway stents can reduce cancer relapse, though this has not yet been tested on human subjects (99).
Therapeutic bronchoscopy for peripheral lung cancer
There are two primary reasons for treating peripheral lung cancer bronchoscopically. The first is to address early-stage cancer with curative intent in patients who are not suitable candidates for surgery or who choose not to undergo surgery. The second reason is to stimulate immune responses by administering local treatments to either the primary tumor or lymph nodes, which can potentially assist with subsequent treatments.
Ablative therapy
A significant body of research has been dedicated to the local treatment of peripheral lung cancer, primarily through percutaneous computed tomography (CT)-guided therapy. However, there are two major concerns regarding complications associated with percutaneous CT-guided procedures in thoracic malignancies—pneumothorax and pleural seeding. A review article published in 2014 reported an average pneumothorax rate of 20% (ranging from 9–54%) from transthoracic needle biopsy (100,101). Additionally, a meta-analysis demonstrated an increased risk of ipsilateral pleural recurrence, with a hazard ratio of 2.58 [confidence interval (CI): 1.15–5.78], when comparing transthoracic biopsy with other diagnostic procedures (100,102). Although the data from transthoracic percutaneous biopsy cannot be directly extrapolated to therapy, there are shared concerns. Advancements in bronchoscopic navigation, such as augmented fluoroscopy and advanced intraprocedural cross-sectional imaging, have made bronchoscopic treatment of peripheral lung cancer a feasible option. This approach potentially mitigates the aforementioned concerns as the procedure theoretically involves fewer violations of the pleura. Currently, research in this area is still in the early stage of development.
Several ablative modalities mentioned above, such as MWA, PDT, cryotherapy, and brachytherapy, have been investigated for the ablation of peripheral lung cancer.
Initially, MWA for lung tumors was primarily studied in the percutaneous setting. A meta-analysis demonstrated promising results with percutaneous MWA treatment for lung cancer, showing an overall survival of 79.3% and a progression-free survival of 64.7% at 1 year (103). The review reported complications, such as pneumothorax (33.9% rate, CI: 23.8–44.8%) and pleural effusion (9.6% rate, CI: 1.5–22.4%). More recently MWA via bronchoscopy has been investigated to improve safety. Chan et al. investigated transbronchial MWA with electromagnetic navigation bronchoscopy, reporting a 100% technical success rate with no progression during a median follow-up of 12 months (104). Complications included pain, pneumothorax requiring drainage (6.67%), post-ablation syndrome, pleural effusion, and hemoptysis. Lau et al. reported their 3.5-year experience of bronchoscopic MVA, including primary lung cancer and oligometastatic nodules with no 30-day mortality or pneumothorax (105). After a median follow-up of 404 days, 20% patients developed local recurrence of the ablated nodule. A more recent study focused on the safety and feasibility of the NEUWAVETM FLEX MWA system (Ethicon Inc., Raritan, NJ, USA). It demonstrated 100% technical success and technique efficacy in all 10 patients with 11 tumors (10 NSCLC, 1 carcinoid), using navigational bronchoscopy guided by cone-beam CT with augmented fluoroscopy (106). Two serious adverse events occurred within 30 days, including a COPD exacerbation and a death of unknown cause. The NEUWAVE FLEX MWA system has been reported to be safely used in animal models for single or multiple MWAs, in conjunction with robotic-assisted bronchoscopy (RAB) (107). A prospective multicenter single-arm study using this MWA system and RAB is currently underway (POWER study).
CT-guided transthoracic PDT was examined in a study involving nine patients (108). Seven out of nine patients achieved partial remission, and two cases of pneumothorax was reported. Chen et al. treated three patients with peripheral lung cancer using PDT bronchoscopically via electromagnetic navigation bronchoscopy (109). All nodules were successfully ablated, and follow-up CT scans demonstrated significant tumor shrinkage. No major procedure-related complications occurred. Additionally, a recently published case report described navigational bronchoscopy-guided PDT for a patient with early-stage peripheral lung cancer, who underwent lobectomy on day 30 and subsequent pathology revealed no viable remaining tumor (110).
Lung parenchymal cryotherapy has also been widely adopted using the percutaneous approach. In one prospective study, 217 CT-guided percutaneous cryotherapy sessions were conducted in 187 patients (111). CT scans identified ice formation, with a mean coverage of 99% for peripheral masses ≤4 cm and 80% for central masses >4 cm. After 6 months, 86% of masses showed reduced or stable size. The incidence of pneumothorax was reported at 12%. In contrast, the application of bronchoscopic cryotherapy for the treatment of lung cancer is currently limited to animal studies. One such study concluded that a flexible cryoprobe using a bronchoscope in ex viva pig lung demonstrated a sufficiently safe treatment method (112).
Brachytherapy can be performed either under CT guidance or bronchoscopically using a catheter. One advantage of bronchoscopic therapy over CT-guided therapy is that the catheter with radioactive seeds can be fixed and left in place for several days, allowing for the implementation of highly fractioned irradiation schedules (113). In a prospective feasibility trial, a patient with right upper lobe lung cancer received combined external-beam radiotherapy and brachytherapy via electromagnetic navigational bronchoscopy (113). The brachytherapy catheter was well tolerated for 5 days. During the 12-month follow-up, CT scans showed partial remission while repeated biopsies confirmed complete remission. Another study reported two cases of bronchoscopic high dose rate (HDR) brachytherapy (114). In this study, barium was injected as a marker and 7 days later, 192Ir was inserted using the HDR after-loading system. CT scans demonstrated focal radiation fibrosis at the treatment site and no change in tumor size in 18 months in the one patient, while the other patient experienced a 75% decrease in tumor size in 10 months.
In addition to the ablative therapies mentioned above, three other modalities, radiofrequency ablation (RFA), bronchoscopic thermal vapor ablation (BTVA), and pulsed electric field (PEF), have also been utilized for the treatment of peripheral lung cancer.
RFA employs an electromagnetic wave to generate a high frequency current for heating and coagulating tissues. Percutaneous CT-guided thermal ablation of stage I–II NSCLC has been shown to be effective (115-117). A systematic review indicated a median complete necrosis rate of 90% with a 1-year survival rate of 63–85% (118). A meta-analysis reported an overall survival of 89.2% and a progression-free survival of 62.4% in 1 year (103). Pneumothorax was the most common complication associated with CT-guided RFA, reported up to 57% of previous studies (115-117). To address this, bronchoscopic RFA catheters have been developed. Koizumi et al. performed bronchoscopy-guided RFA in 20 patients with stage IA–IB diseases, reporting a median progression-free survival of 35 months (95% CI: 22–45) with no reported incidents of pneumothorax (119). Xie et al. utilized a novel RFA probe with a ‘flower-like’ appearance to increase the treatment volume of the probe, achieving either partial or complete response in all three patients with no significant complications (120). However, the interest in researching RFA has diminished due to the reported advantages of MWA over RFA. These advantages include shorter procedural time, reduced heat-sink effect, large ablation zones, decreased susceptibility to tissue impedance and the ability to use multiple antennae simultaneously (121).
Another modality, BTVA is being explored in the local treatment of peripheral lung cancer after its initial inception to treat emphysema (122). This technique delivers steam that travels along air-filled spaces, confining to the anatomical boundaries of the subsegment of the treated lung parenchyma (123). In a study of six subjects with peripheral lung cancers who received BTVA, 5 out of 6 patients received surgical resection and pathological examination showed complete necrosis in two of the patients (124). Two subjects reported post-procedural pleuritic chest pain.
PEF is a nonthermal ablative modality that induces electroporation by utilizing strong pulsed electrical fields to create microscopic pores in cell membranes. The Aliya system (Galvanize Therapeutics, GTI-00018 investigational device, San Carlos, CA, USA) is a commercially available biphasic monopolar PEF system that uses a single percutaneous probe to target a lesion of interest. This process ultimately leads to a change in membrane permeability and necrosis (125). Additionally, local PEF has demonstrated the ability to induce immunogenic cell death and increase T cell infiltration in tumors, and the combination of PEF with immune checkpoint inhibitor therapy has shown a synergistic immunostimulatory cytokine profile (126). A porcine model has confirmed the safety and histological efficacy of bronchoscopic delivery of PEF in lung tissue (127). There is a forthcoming ablate and resect clinical trial designed to further delineate the treatment effects of PEF in-vivo when used either percutaneously or via bronchoscopy. ClinicalTrials.gov identifier: NCT04732520. Updated January 9, 2023. Accessed July 1, 2023. https://classic.clinicaltrials.gov/ct2/show/NCT04732520.
Peripheral tumor injection of chemotherapy or gene therapies
Intratumoral injection of chemotherapy theoretically increases local drug level while minimizing systemic toxicity (128). Cisplatin is the most frequently used agent in the previous studies focused on the central airway and lymph nodes (129-131). Due to the uncertainties surrounding patient selection, optimal dosage and long-term outcomes, the adoption of these approaches in clinical practice has been limited.
Bronchoscopic delivery of therapeutic genes for lung cancer patients has also been investigated. These studies aim to investigate the role of local therapy in cancer immune response. A phase I trial reported intratumoral injection of CCL21 gene-modified dendritic cells for patients with stage IIIB/IV NSCLC, results in induction of systemic tumor antigen-specific immune response and increased tumor PD-L1 expression (132). Additionally, bronchoscopic delivery of a recombinant adenovirus carrying the wild-type p53 gene was given to patients with NSCLC in combination with radiotherapy (133). In 63% of patients, biopsy-proven nonviable tumor was observed. Another phase I trial investigated the use of an adenovirus vector containing the HSV-tk gene (AdV-tk) in patients with resectable NSCLC (134). The use of bronchoscopic AdV-tK injection resulted in minimal vector-related adverse events and a significantly increase in infiltration of CD8+ T cells in surgically resected tumors.
Conclusions
In summary, there are various bronchoscopic management modalities available for the treatment of MCAO, each with its own unique characteristics that need to be considered in clinical applications. Interventional pulmonologists often combine different modalities to achieve the most satisfactory results. Anesthesia and airway management can pose challenges, and the choice typically depends on the patient’s clinical stability and the selected bronchoscopic modality. For early-stage, airway limited malignancies, curative bronchoscopic treatments are available. Furthermore, emerging treatment options are being explored. Interventional pulmonology has also expanded its scope to include local treatment of peripheral lung cancer. However, additional research is needed to establish its efficacy and potential benefits of these interventions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-102/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-102/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. DD received research grants to institution from Gala Therapeutics and Auris Medical, Inc.; and received personal consulting fees from Olympus, Johnson & Johnson, Intuitive Surgical and Auris Medical, Inc. KM received research grants to institution from Right Air, Inc. and Pulmonx; received personal consulting fees from Pulmonx; and served as an advisory board member of Polarean, Inc. and Verona Pharmaceutical (paid). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ost DE, Ernst A, Grosu HB, et al. Therapeutic bronchoscopy for malignant central airway obstruction: success rates and impact on dyspnea and quality of life. Chest 2015;147:1282-98. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Shiau M, Harkin TJ, Naidich DP. Imaging of the central airways with bronchoscopic correlation: pictorial essay. Clin Chest Med 2015;36:313-34. ix-x. [Crossref] [PubMed]
- Walser EM, Robinson B, Raza SA, et al. Clinical outcomes with airway stents for proximal versus distal malignant tracheobronchial obstructions. J Vasc Interv Radiol 2004;15:471-7. [Crossref] [PubMed]
- Shin B, Chang B, Kim H, et al. Interventional bronchoscopy in malignant central airway obstruction by extra-pulmonary malignancy. BMC Pulm Med 2018;18:46. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Uncommon primary tracheal tumors. Ann Thorac Surg 2006;82:268-72; discussion 272-3. [Crossref] [PubMed]
- Myer CM 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 1994;103:319-23. [Crossref] [PubMed]
- Chaddha U, Hogarth DK, Murgu S. Bronchoscopic Ablative Therapies for Malignant Central Airway Obstruction and Peripheral Lung Tumors. Ann Am Thorac Soc 2019;16:1220-9. [Crossref] [PubMed]
- Stratakos G, Gerovasili V, Dimitropoulos C, et al. Survival and Quality of Life Benefit after Endoscopic Management of Malignant Central Airway Obstruction. J Cancer 2016;7:794-802. [Crossref] [PubMed]
- Razi SS, Lebovics RS, Schwartz G, et al. Timely airway stenting improves survival in patients with malignant central airway obstruction. Ann Thorac Surg 2010;90:1088-93. [Crossref] [PubMed]
- Jung HS, Chae G, Kim JH, et al. The mechanical characteristics and performance evaluation of a newly developed silicone airway stent (GINA stent). Sci Rep 2021;11:7958. [Crossref] [PubMed]
- Podgaetz E, Harmon K, Andrade RS, et al. Endobronchial Excision of a Large Near-Occlusive Tracheal Tumor. Operative and Anesthetic Considerations. Ann Am Thorac Soc 2015;12:1881-4. [Crossref] [PubMed]
- Payne CB Jr, Goyal PC, Gupta SC. Effects of transoral and transnasal fiberoptic bronchoscopy on oxygenation and cardiac rhythm. Endoscopy 1986;18:1-3. [Crossref] [PubMed]
- Morrison MP, Meiler S, Postma GN. Ventilatory techniques for central airway obstruction. Laryngoscope 2011;121:2162-4. [Crossref] [PubMed]
- Conacher ID, Paes LL, McMahon CC, et al. Anesthetic management of laser surgery for central airway obstruction: a 12-year case series. J Cardiothorac Vasc Anesth 1998;12:153-6. [Crossref] [PubMed]
- Du Rand IA, Barber PV, Goldring J, et al. British Thoracic Society guideline for advanced diagnostic and therapeutic flexible bronchoscopy in adults. Thorax 2011;66:iii1-21. [Crossref] [PubMed]
- Lee PM, Malhotra R, Shojaee S, et al. Therapeutic Strategies and Ventilatory Management during Interventional Rigid Bronchoscopy for Malignant Central Airway Obstruction. Ann Am Thorac Soc 2016;13:574-5. [Crossref] [PubMed]
- Okamoto S, Somiya N, M, Saito A, et al. A Prospective, Randomized Trial Comparing Respiratory Status During Anesthesia for Airway Stenting: Spontaneous Respiration Versus Controlled Ventilation With Muscle Relaxants. Anesth Analg 2020;131:893-900. [Crossref] [PubMed]
- Hong Y, Jo KW, Lyu J, et al. Use of venovenous extracorporeal membrane oxygenation in central airway obstruction to facilitate interventions leading to definitive airway security. J Crit Care 2013;28:669-74. [Crossref] [PubMed]
- Pertzov B, Gershman E, Izhakian S, et al. Placement of self-expanding metallic tracheobronchial Y stent with laryngeal mask airway using conscious sedation under fluoroscopic guidance. Thorac Cancer 2021;12:484-90. [Crossref] [PubMed]
- Chen X, Zhou Y, Yu H, et al. Feasibility analysis of flexible bronchoscopy in conjunction with noninvasive ventilation for therapy of hypoxemic patients with Central Airway Obstruction: a retrospective study. PeerJ 2020;8:e8687. [Crossref] [PubMed]
- Cheng LTW, Sim TB, Kuan WS. Noninvasive Ventilation as a Temporizing Measure in Critical Fixed Central Airway Obstruction: A Case Report. J Emerg Med 2018;54:615-8. [Crossref] [PubMed]
- Chen CH, Wu BR, Cheng WC, et al. Interventional pulmonology for patients with central airway obstruction: An 8-year institutional experience. Medicine (Baltimore) 2017;96:e5612. [Crossref] [PubMed]
- Mahmood K, Wahidi MM. Ablative therapies for central airway obstruction. Semin Respir Crit Care Med 2014;35:681-92. [Crossref] [PubMed]
- Tremblay A, Marquette CH. Endobronchial electrocautery and argon plasma coagulation: a practical approach. Can Respir J 2004;11:305-10. [Crossref] [PubMed]
- van Boxem TJ, Westerga J, Venmans BJ, et al. Tissue effects of bronchoscopic electrocautery: bronchoscopic appearance and histologic changes of bronchial wall after electrocautery. Chest 2000;117:887-91. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-83. [Crossref] [PubMed]
- Senitko M, Oberg CL, Abraham GE, et al. Microwave Ablation for Malignant Central Airway Obstruction: A Pilot Study. Respiration 2022;101:666-74. [Crossref] [PubMed]
- Dou JP, Yu J, Yang XH, et al. Outcomes of microwave ablation for hepatocellular carcinoma adjacent to large vessels: a propensity score analysis. Oncotarget 2017;8:28758-68. [Crossref] [PubMed]
- Sheski FD, Mathur PN. Endobronchial electrosurgery: argon plasma coagulation and electrocautery. Semin Respir Crit Care Med 2004;25:367-74. [Crossref] [PubMed]
- Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994;2:42-6. [PubMed]
- Shaller BD, Filsoof D, Pineda JM, et al. Malignant Central Airway Obstruction: What's New? Semin Respir Crit Care Med 2022;43:512-29. [Crossref] [PubMed]
- McDougall JC, Cortese DA. Neodymium-YAG laser therapy of malignant airway obstruction. A preliminary report. Mayo Clin Proc 1983;58:35-9. [PubMed]
- Mudambi L, Miller R, Eapen GA. Malignant central airway obstruction. J Thorac Dis 2017;9:S1087-110. [Crossref] [PubMed]
- Ernst A, Wahidi MM, Read CA, et al. Adult Bronchoscopy Training: Current State and Suggestions for the Future: CHEST Expert Panel Report. Chest 2015;148:321-32. [Crossref] [PubMed]
- Folch E, Mehta AC. Airway interventions in the tracheobronchial tree. Semin Respir Crit Care Med 2008;29:441-52. [Crossref] [PubMed]
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc 2016;13:1405-15. [Crossref] [PubMed]
- Aboudara M, Rickman O, Maldonado F. Therapeutic Bronchoscopic Techniques Available to the Pulmonologist: Emerging Therapies in the Treatment of Peripheral Lung Lesions and Endobronchial Tumors. Clin Chest Med 2020;41:145-60. [Crossref] [PubMed]
- Browning R, Turner JF Jr, Parrish S. Spray cryotherapy (SCT): institutional evolution of techniques and clinical practice from early experience in the treatment of malignant airway disease. J Thorac Dis 2015;7:S405-14. [PubMed]
- Moore RF, Lile DJ, Abbas AE. Current status of spray cryotherapy for airway disease. J Thorac Dis 2017;9:S122-9. [Crossref] [PubMed]
- Hayata Y, Kato H, Konaka C, et al. Hematoporphyrin derivative and laser photoradiation in the treatment of lung cancer. Chest 1982;81:269-77. [Crossref] [PubMed]
- Kato H, Usuda J, Okunaka T, et al. Basic and clinical research on photodynamic therapy at Tokyo Medical University Hospital. Lasers Surg Med 2006;38:371-5. [Crossref] [PubMed]
- Usuda J, Kato H, Okunaka T, et al. Photodynamic therapy (PDT) for lung cancers. J Thorac Oncol 2006;1:489-93. [Crossref] [PubMed]
- Ikeda N, Usuda J, Maehara S. Photodynamic therapy for central-type early-stage lung cancer. Gen Thorac Cardiovasc Surg 2020;68:679-83. [Crossref] [PubMed]
- Triesscheijn M, Baas P, Schellens JH, et al. Photodynamic therapy in oncology. Oncologist 2006;11:1034-44. [Crossref] [PubMed]
- Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71. [Crossref] [PubMed]
- Vergnon JM, Huber RM, Moghissi K. Place of cryotherapy, brachytherapy and photodynamic therapy in therapeutic bronchoscopy of lung cancers. Eur Respir J 2006;28:200-18. [Crossref] [PubMed]
- Khanavkar B, Stern P, Alberti W, et al. Complications associated with brachytherapy alone or with laser in lung cancer. Chest 1991;99:1062-5. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;7:273-83. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4. [Crossref] [PubMed]
- Lee P, Kupeli E, Mehta AC. Airway stents. Clin Chest Med 2010;31:141-50. Table of Contents. [Crossref] [PubMed]
- Dutau H, Di Palma F, Thibout Y, et al. Impact of Silicone Stent Placement in Symptomatic Airway Obstruction due to Non-Small Cell Lung Cancer - A French Multicenter Randomized Controlled Study: The SPOC Trial. Respiration 2020;99:344-52. [Crossref] [PubMed]
- Dutau H, Breen D, Bugalho A, et al. Current Practice of Airway Stenting in the Adult Population in Europe: A Survey of the European Association of Bronchology and Interventional Pulmonology (EABIP). Respiration 2018;95:44-54. [Crossref] [PubMed]
- Mathew R, Hibare K, Dalar L, et al. Tracheobronchial stent sizing and deployment practices airway stenting practices around the world: a survey study. J Thorac Dis 2020;12:5495-504. [Crossref] [PubMed]
- Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest 1998;114:106-9. [Crossref] [PubMed]
- Noppen M, Poppe K, D'Haese J, et al. Interventional bronchoscopy for treatment of tracheal obstruction secondary to benign or malignant thyroid disease. Chest 2004;125:723-30. [Crossref] [PubMed]
- Profili S, Manca A, Feo CF, et al. Palliative airway stenting performed under radiological guidance and local anesthesia. Cardiovasc Intervent Radiol 2007;30:74-8. [Crossref] [PubMed]
- Gildea TR, Young BP, Machuzak MS. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Özgül MA, Çetinkaya E, Seyhan EC, et al. Airway stents: a retrospective evaluation of indications, results and complications in our 10-year experience. Tuberk Toraks 2019;67:272-84. [Crossref] [PubMed]
- Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9. [Crossref] [PubMed]
- Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest 2012;141:1473-81. [Crossref] [PubMed]
- Lemaire A, Burfeind WR, Toloza E, et al. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann Thorac Surg 2005;80:434-7; discussion 437-8. [Crossref] [PubMed]
- Saito Y, Imamura H. Airway stenting. Surg Today 2005;35:265-70. [Crossref] [PubMed]
- Oki M, Handa H, Saka H, et al. Changes in Pulmonary Function Test Results and Respiratory Symptoms before and after Airway Stent Removal. Respiration 2022;101:925-30. [Crossref] [PubMed]
- Lee BR, Oh IJ, Lee HS, et al. Usefulness of Rigid Bronchoscopic Intervention Using Argon Plasma Coagulation for Central Airway Tumors. Clin Exp Otorhinolaryngol 2015;8:396-401. [Crossref] [PubMed]
- Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-76. [Crossref] [PubMed]
- Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg 2001;19:339-45. [Crossref] [PubMed]
- Manninen MP, Antila PJ, Pukander JS, et al. Occurrence of tracheal carcinoma in Finland. Acta Otolaryngol 1991;111:1162-9. [Crossref] [PubMed]
- Chan AL, Shelton DK, Yoneda KY. Unusual primary lung neoplasms. Curr Opin Pulm Med 2001;7:234-41. [Crossref] [PubMed]
- Zellweger M, Grosjean P, Goujon D, et al. In vivo autofluorescence spectroscopy of human bronchial tissue to optimize the detection and imaging of early cancers. J Biomed Opt 2001;6:41-51. [Crossref] [PubMed]
- Shibuya K, Hoshino H, Chiyo M, et al. Subepithelial vascular patterns in bronchial dysplasias using a high magnification bronchovideoscope. Thorax 2002;57:902-7. [Crossref] [PubMed]
- Zaric B, Perin B, Stojsic V, et al. Relation between vascular patterns visualized by Narrow Band Imaging (NBI) videobronchoscopy and histological type of lung cancer. Med Oncol 2013;30:374. [Crossref] [PubMed]
- Fuchs FS, Zirlik S, Hildner K, et al. Confocal laser endomicroscopy for diagnosing lung cancer in vivo. Eur Respir J 2013;41:1401-8. [Crossref] [PubMed]
- Takahashi H, Handa M, Oyaizu A, et al. Ultrasonographical approach for the diagnosis on the depth of invasion in early bronchogenic squamous cell carcinoma. Kyobu Geka 2001;54:907-12. [PubMed]
- Takahashi H, Sagawa M, Sato M, et al. A prospective evaluation of transbronchial ultrasonography for assessment of depth of invasion in early bronchogenic squamous cell carcinoma. Lung Cancer 2003;42:43-9. [Crossref] [PubMed]
- Guibert N, Mhanna L, Droneau S, et al. Techniques of endoscopic airway tumor treatment. J Thorac Dis 2016;8:3343-60. [Crossref] [PubMed]
- Fujimura S, Sakurada A, Sagawa M, et al. A therapeutic approach to roentgenographically occult squamous cell carcinoma of the lung. Cancer 2000;89:2445-8. [Crossref] [PubMed]
- Corti L, Toniolo L, Boso C, et al. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 2007;39:394-402. [Crossref] [PubMed]
- Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J 2003;22:535-41. [Crossref] [PubMed]
- Hayata Y, Kato H, Konaka C, et al. Photodynamic therapy (PDT) in early stage lung cancer. Lung Cancer 1993;9:287-93. [Crossref]
- Furuse K, Fukuoka M, Kato H, et al. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. The Japan Lung Cancer Photodynamic Therapy Study Group. J Clin Oncol 1993;11:1852-7. [Crossref] [PubMed]
- Kato H. Photodynamic therapy for lung cancer--a review of 19 years' experience. J Photochem Photobiol B 1998;42:96-9. [Crossref] [PubMed]
- Ono R, Ikeda S, Suemasu K. Hematoporphyrin derivative photodynamic therapy in roentgenographically occult carcinoma of the tracheobronchial tree. Cancer 1992;69:1696-701. [Crossref] [PubMed]
- Kato H, Konaka C, Ono J, et al. Preoperative laser photodynamic therapy in combination with operation in lung cancer. J Thorac Cardiovasc Surg 1985;90:420-9. [Crossref] [PubMed]
- Deygas N, Froudarakis M, Ozenne G, et al. Cryotherapy in early superficial bronchogenic carcinoma. Chest 2001;120:26-31. [Crossref] [PubMed]
- Brokx HA, Risse EK, Paul MA, et al. Initial bronchoscopic treatment for patients with intraluminal bronchial carcinoids. J Thorac Cardiovasc Surg 2007;133:973-8. [Crossref] [PubMed]
- Luckraz H, Amer K, Thomas L, et al. Long-term outcome of bronchoscopically resected endobronchial typical carcinoid tumors. J Thorac Cardiovasc Surg 2006;132:113-5. [Crossref] [PubMed]
- Sutedja TG, Schreurs AJ, Vanderschueren RG, et al. Bronchoscopic therapy in patients with intraluminal typical bronchial carcinoid. Chest 1995;107:556-8. [Crossref] [PubMed]
- Bertoletti L, Elleuch R, Kaczmarek D, et al. Bronchoscopic cryotherapy treatment of isolated endoluminal typical carcinoid tumor. Chest 2006;130:1405-11. [Crossref] [PubMed]
- Homasson JP, Thiery JP, Angebault M, et al. The operation and efficacy of cryosurgical, nitrous oxide-driven cryoprobe. I. Cryoprobe physical characteristics: their effects on cell cryodestruction. Cryobiology 1994;31:290-304. [Crossref] [PubMed]
- van Boxem TJ, Venmans BJ, Schramel FM, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72. [Crossref] [PubMed]
- Cavaliere S, Foccoli P, Toninelli C, et al. Nd: YAG laser therapy in lung cancer: an 11-year experience with 2,253 applications in 1,585 patients. J Bronchol Int Pulmonol 1994;1:105-11. [Crossref]
- Aumont-le Guilcher M, Prevost B, Sunyach MP, et al. High-dose-rate brachytherapy for non-small-cell lung carcinoma: a retrospective study of 226 patients. Int J Radiat Oncol Biol Phys 2011;79:1112-6. [Crossref] [PubMed]
- Gafford JB, Webster S, Dillon N, et al. A Concentric Tube Robot System for Rigid Bronchoscopy: A Feasibility Study on Central Airway Obstruction Removal. Ann Biomed Eng 2020;48:181-91. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Restoration of Patency to Central Airways Occluded by Malignant Endobronchial Tumors Using Intratumoral Injection of Cisplatin. Ann Am Thorac Soc 2015;12:1345-50. [Crossref] [PubMed]
- Guan WJ, Li SY, Zhong NS. Effects of para-toluenesulfonamide intratumoral injection on pulmonary adenoid cystic carcinoma complicating with severe central airway obstruction: a 5-year follow-up study. J Thorac Dis 2018;10:2448-55. [Crossref] [PubMed]
- Zhou Y, Gao Y, Zhang N, et al. Clinical effects of cisplatin plus recombinant human endostatin (rh-endostatin) intratumoral injection on malignant central airway obstruction: a retrospective analysis of 319 cases. J Thorac Dis 2021;13:1100-5. [Crossref] [PubMed]
- Xu J, Ong HX, Traini D, et al. Paclitaxel-eluting silicone airway stent for preventing granulation tissue growth and lung cancer relapse in central airway pathologies. Expert Opin Drug Deliv 2020;17:1631-45. [Crossref] [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6:S99-S107. [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Hong H, Hahn S, Matsuguma H, et al. Pleural recurrence after transthoracic needle lung biopsy in stage I lung cancer: a systematic review and individual patient-level meta-analysis. Thorax 2021;76:582-90. [Crossref] [PubMed]
- Yuan Z, Wang Y, Zhang J, et al. A Meta-Analysis of Clinical Outcomes After Radiofrequency Ablation and Microwave Ablation for Lung Cancer and Pulmonary Metastases. J Am Coll Radiol 2019;16:302-14. [Crossref] [PubMed]
- Chan JWY, Lau RWH, Ngai JCL, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance-a novel technique and initial experience with 30 cases. Transl Lung Cancer Res 2021;10:1608-22. [Crossref] [PubMed]
- Lau K, Maceviciute K, Baranowski R. Long Term Outcomes of Bronchoscopic Microwave Ablation of Peripheral Lung Nodules. In: B110 The MIDAS Touch Interventional Pulmonology in Thoracic Oncology. Am J Respir Crit Care Med 2022;205:A3675.
- Pritchett MA, Reisenauer JS, Kern R, et al. Novel Image-Guided Flexible-Probe Transbronchial Microwave Ablation for Stage 1 Lung Cancer. Respiration 2023;102:182-93. [Crossref] [PubMed]
- De Leon H, Royalty K, Mingione L, et al. Device safety assessment of bronchoscopic microwave ablation of normal swine peripheral lung using robotic-assisted bronchoscopy. Int J Hyperthermia 2023;40:2187743. [Crossref] [PubMed]
- Okunaka T, Kato H, Tsutsui H, et al. Photodynamic therapy for peripheral lung cancer. Lung Cancer 2004;43:77-82. [Crossref] [PubMed]
- Chen KC, Lee JM. Photodynamic therapeutic ablation for peripheral pulmonary malignancy via electromagnetic navigation bronchoscopy localization in a hybrid operating room (OR): a pioneering study. J Thorac Dis 2018;10:S725-30. [Crossref] [PubMed]
- Allison RR, Bansal S. Photodynamic therapy for peripheral lung cancer. Photodiagnosis Photodyn Ther 2022;38:102825. [Crossref] [PubMed]
- Wang H, Littrup PJ, Duan Y, et al. Thoracic masses treated with percutaneous cryotherapy: initial experience with more than 200 procedures. Radiology 2005;235:289-98. [Crossref] [PubMed]
- Zheng X, Yang C, Zhang X, et al. The Cryoablation for Peripheral Pulmonary Lesions Using a Novel Flexible Bronchoscopic Cryoprobe in the ex vivo Pig Lung and Liver. Respiration 2019;97:457-62. [Crossref] [PubMed]
- Harms W, Krempien R, Grehn C, et al. Electromagnetically navigated brachytherapy as a new treatment option for peripheral pulmonary tumors. Strahlenther Onkol 2006;182:108-11. [Crossref] [PubMed]
- Kobayashi T, Kaneko M, Sumi M, et al. CT-assisted transbronchial brachytherapy for small peripheral lung cancer. Jpn J Clin Oncol 2000;30:109-12. [Crossref] [PubMed]
- Grieco CA, Simon CJ, Mayo-Smith WW, et al. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol 2006;17:1117-24. [Crossref] [PubMed]
- Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg 2007;134:1306-12. [Crossref] [PubMed]
- Jin GY, Han YM, Lee YS, et al. Radiofrequency ablation using a monopolar wet electrode for the treatment of inoperable non-small cell lung cancer: a preliminary report. Korean J Radiol 2008;9:140-7. [Crossref] [PubMed]
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 2008;15:1765-74. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Xie F, Zheng X, Xiao B, et al. Navigation Bronchoscopy-Guided Radiofrequency Ablation for Nonsurgical Peripheral Pulmonary Tumors. Respiration 2017;94:293-8. [Crossref] [PubMed]
- Ni Y, Xu H, Ye X. Image-guided percutaneous microwave ablation of early-stage non-small cell lung cancer. Asia Pac J Clin Oncol 2020;16:320-5. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [Crossref] [PubMed]
- Kramer T, Annema JT. Advanced bronchoscopic techniques for the diagnosis and treatment of peripheral lung cancer. Lung Cancer 2021;161:152-62. [Crossref] [PubMed]
- Steinfort DP, Christie M, Antippa P, et al. Bronchoscopic Thermal Vapour Ablation for Localized Cancer Lesions of the Lung: A Clinical Feasibility Treat-and-Resect Study. Respiration 2021;100:432-42. [Crossref] [PubMed]
- Anic A, Breskovic T, Sikiric I. Pulsed field ablation: a promise that came true. Curr Opin Cardiol 2021;36:5-9. [Crossref] [PubMed]
- Silvestrini M, Pastori C, Tamakloe S, et al. EP02. 04-002 Synergy of Local Treatment with Pulsed Electric Fields and Anti-PD1 Checkpoint Blockade. J Thorac Oncol 2022;17:S232. [Crossref]
- O'Brien T, Krimsky W, Neal R. The Safety of Transbronchial and Percutaneous Delivery of Pulsed Electric Fields in Lung. In: A109 The Odyssey, No Longer A Tragedy: The Continuum Of Lung Cancer. Am J Respir Crit Care Med 2022;205:A5561.
- Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer 2008;61:1-12. [Crossref] [PubMed]
- Jiang W, Yang X, Wang X, et al. Bronchoscopic intratumoral injections of cisplatin and endostar as concomitants of standard chemotherapy to treat malignant central airway obstruction. Postgrad Med J 2022;98:104-12. [Crossref] [PubMed]
- Jabbardarjani H, Kharabian S, Masjedi MR. Endobronchial chemotherapy in malignant airway lesions of the lung: report of 3 years experience. J Bronchology Interv Pulmonol 2007;14:242-5.
- DuComb EA, Collins CC, Cupak D, et al. Endobronchial ultrasound-guided transbronchial needle injection of cisplatin results in dynamic changes in the tumor immune microenvironment. Respir Med Res 2023;84:100994. [Crossref] [PubMed]
- Lee JM, Lee MH, Garon E, et al. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8(+) T-cell Infiltration. Clin Cancer Res 2017;23:4556-68. [Crossref] [PubMed]
- Swisher SG, Roth JA, Komaki R, et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res 2003;9:93-101. [PubMed]
- Predina JD, Haas AR, Martinez M, et al. Neoadjuvant Gene-Mediated Cytotoxic Immunotherapy for Non-Small-Cell Lung Cancer: Safety and Immunologic Activity. Mol Ther 2021;29:658-70. [Crossref] [PubMed]
Cite this article as: Zhang C, Dibardino DM, Ma KC. Bronchoscopic treatment of thoracic malignancy. AME Med J 2023;8:36.