
Benign airway obstruction: a clinical practice review of causes and managements principles
Introduction
Central airway obstruction (CAO) is defined as narrowing of the central airways: the subglottic space, trachea, right and left mainstem bronchi, and right bronchus intermedius. Obstruction in more distal airways, lobar bronchi and beyond, is peripheral airway obstruction. In addition to airway location, airway obstruction is classified by pathology as malignant or non-malignant, with malignant causes being more common and associated with worse outcomes (1). In this review, we will discuss the causes and management strategies for non-malignant airway obstruction, also referred to as benign airway obstruction. Though benign, this group of diseases can have potentially devastating consequences and consists of diverse pathology and clinical presentations. In contrast with malignant airway obstruction, the diseases leading to benign CAO are often not life-limiting and the assessment and management of airway obstruction for these patients must account for this. Despite the lack of large, randomized clinical trials, there have been advancements in treatment, both procedural and nonprocedural, available for benign CAO. Multiple types of specialists, including pulmonologists, otolaryngologists, and thoracic surgeons, may manage patients with benign airway obstruction and have contributed to the growing body of research and literature available to understand and manage these patients. This review will focus on unifying knowledge across these diverse fields and identifying areas for collaborative management and further study.
Causes and classification
Numerous diseases cause benign airway obstruction via varying mechanisms (Table 1) and it is paramount to identify the distinct cause and pathophysiology of airway stenosis in each patient. Several classification systems have been used to describe CAO in general or for specific diseases. These systems include both structural descriptors of the stenosis as well as functional or symptomatic scores. For example, the original and expanded Myer-Cotton grading systems, developed by laryngologists for subglottic stenosis, utilizes the percentage of obstruction, the extent of disease, based on number of sites involved, as well as comorbidities with higher scores correlating with worse surgical or functional outcomes (2,3). A classification schema for any etiology of tracheobronchial stenosis proposed by Freitag and colleagues proposed simple and reproducible classification by the type, degree, and location of stenos(es) (4). However, no classification systems have yet been widely adopted.
Table 1
| Type of stenosis | Examples |
|---|---|
| Intraluminal | Inflammatory and autoimmune (granulomatosis with polyangiitis, relapsing polychondritis, sarcoidosis, amyloidosis) |
| Infections (tuberculosis, fungal infections including aspergillosis and blastomycosis, HPV-related respiratory papillomatosis) | |
| Benign tumor (hemangioma, hamartoma, tracheopathia osteoplastica, granulation tissue) | |
| Endogenous (mucus plug, blood clot, broncholith) | |
| Exogenous foreign body | |
| Extrinsic compression | Lymphadenopathy, goiter |
| Scar/stricture | Post intubation/tracheostomy tracheal stenosis, idiopathic subglottic stenosis |
| Distortion | Vascular sling, post-pneumonectomy distortion |
| Post surgical complications | Post lung transplant, post sleeve resection, post airway stenting |
| Dynamic airway obstruction | TBM, EDAC |
HPV, human papillomavirus; TBM, tracheobronchomalacia; EDAC, excessive dynamic airway collapse.
Presentation
Patient presentation and symptoms depend on the location, acuity, and extent of obstruction as well as patient’s underlying cardiopulmonary reserve. For example, acute airway obstruction, such as foreign body aspiration, is more symptomatic than the typical gradual onset of idiopathic subglottic stenosis. Acuity also influences time to diagnosis: it is not uncommon for patients with gradual onset of symptoms from a chronic, mild, or slowly progressive disease process to be misdiagnosed with asthma or chronic obstructive pulmonary disease (COPD) for months or even years (5,6). It is therefore imperative to maintain a high degree of suspicion, especially if history is suggestive of an alternative diagnosis or there is no response to disease-targeted therapy.
Severity of symptoms, often dyspnea, correlates with diameter and length of the affected airway. Exercise tolerance is affected first, as the pressure gradient across the obstruction increases with the increase of airflow required to increase minute ventilation. In the simplest example, of single site fixed tracheal stenosis, patients typically become dyspneic with exertion when the diameter has narrowed to less than 8 mm (50% stenosis) and experience dyspnea at rest or stridor when the diameter is less than 5 mm (70% stenosis) (5-7). Concomitant other airway or lung diseases, such as asthma, COPD, or indolent infection, should also be considered and can contribute to earlier or atypical symptoms. Key information obtained from patient interviews includes not only their dyspnea symptoms and the course over time, but evaluation of cough symptoms, exercise/activity tolerance, orthopnea, paroxysmal nocturnal dyspnea as well as signs and symptoms of suspected systemic diseases. Key components of the physical exam include auscultation over both the lungs and the airways, oropharynx exam, neck extension, overall functional status, and sequalae of infection or connective tissue disease.
Diagnostic tests
Pulmonary function test with flow volume loops
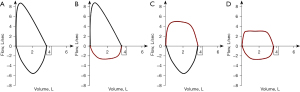
Flow volume loops can categorize CAO into two major groups: fixed and variable obstruction as well as localize disease to intra- or extra-thoracic involvement. Fixed obstruction will have blunting of both inspiratory and expiratory limbs with a box-shaped loop. Variable obstruction can be seen with intrathoracic obstruction presenting with a blunted or flattened expiratory limb and extrathoracic obstruction with blunted or flattened inspiratory limb (Figure 1) (8). Notably, spirometry and flow volume loops have low sensitivity for detection of mild to moderate CAO; the classic blunting of flow volume loops often does not occur until diameter is reduced to 8–10 millimeters (9). Given this, pulmonary function testing should not be used to rule out CAO. Instead, it can be helpful as an adjunctive diagnostic tool. It has also been proven useful in follow up after therapeutic intervention to assess for response or recurrence of obstruction (10).
Imaging
Computed tomography (CT) of the chest is valuable in both diagnosis and treatment planning for benign airway obstruction. Critical information obtained from CT includes site(s), length(s), and severity of stenosis as well as patency of distal airways and identification of non-airway disease. Post-processing of CT imaging can include multiplanar reformation, 3D reconstruction, volume rendering, and creation of virtual bronchoscopy (11-13). For dynamic airway obstruction, comparison of inspiratory and expiratory CT images can utilized to assess degree of expiratory collapse and air-trapping (14). Most recently, CT has allowed for planning for patient-specific, customized airway stenting (15,16).
Bronchoscopy
Bronchoscopy has a potential dual role in benign CAO: diagnostic and therapeutic. Direct visualization of the sites and severity of obstruction along with tissue sampling with needles, brushes, or forceps can be obtained for diagnosis, as well as prognostication and treatment planning. Therapeutically, multiple interventions can be considered including electrosurgery, cryotherapy, light amplification by stimulated emission of radiation (LASER) therapy, mechanical dilation with rigid scopes or balloons, and medication injection or application (17-21). Stenting can also be considered as a second line intervention (22-24) and the therapeutic endoscopy toolkit will be discussed further later in this review.
However, even diagnostic bronchoscopy in CAO is not without risk. At minimum, the bronchoscope further occludes an already narrowed airway with the potential complications of bleeding or mucosal edema causing further obstruction. In patients with CAO, bronchoscopy should be performed by experienced and skilled operators in appropriate facilities with personnel and equipment allowing for complex maneuvers, including rigid bronchoscopy and difficult airway management. The advantages of rigid bronchoscopy include the ability to quickly bypass a critical stenosis as well as rigidly dilate, ventilation via side ports of the rigid barrel, and use of the rigid barrel to secure the airway or enable tamponade when sampling vascular lesions (25).
Management of benign CAO
Management of benign CAO is based on the underlying disease as well as the individual patient, but a few general principles apply to all benign disease involving the central airways. There is no data demonstrating improved survival following endoscopic management of benign CAO. Thus, clinical observation is indicated for mild (less than 50% reduction of lumen) and asymptomatic patients, as well as for patients with severe comorbidities or limitations in functional status that would not improve with intervention (25). For symptomatic patients who are procedural and/or surgical candidates, the clinical scenario must guide the involvement of specialists or subspecialists including otolaryngologists, pulmonologists, interventional pulmonologists, and/or thoracic surgeons. Algorithms developed for tertiary care/academic medical centers have been developed to provide guidance for multidisciplinary approaches for these patients (26,27).
Next, we discuss some common causes of benign airway obstruction and their management, highlighting the available data around management strategies. We have used examples from our practice for illustration.
Post intubation tracheal stenosis and post tracheostomy tracheal stenosis (PITS and PTTS)
Stenosis after an oral, nasal, or surgical airway is the most common cause of acquired benign tracheal stenosis, with an estimated incidence of 4.9 cases per million people per year in the general population (28). PITS has been reported to occurs in anywhere from 1–21% of intubated patients, but with only 1–2% of cases severe enough to warrant intervention (29,30). There is data to suggest this is an under-recognized disease, with as many as four out of five patients with severe PITS having a delayed or missed diagnosis (31). Patient risk factors for both PITS and PTTS have varied between studies, but most frequently include advanced age, female sex, obesity, sleep apnea, use of corticosteroids, acid reflux disease, and history of neck radiation (31-33). Though airway injury can occur after any duration of intubation, risk of PITS increases with higher duration of intubation and use of high endotracheal cuff pressures with cuff pressures exceeding 20 cmH2O lead to trachea ischemia, necrosis, and granulation or scar tissue (34,35). In tracheostomy, abnormal wound healing, including wound infection and cartilaginous damage, are also associated higher incidence of PTTS (34). One study found PTTS to appear to originate from the tracheal stoma 85% of the time, versus the site of the tracheostomy tube cuff seen in PITS (30).
Both PITS and PTTS can be divided into two major categories: simple and complex stenosis. Simple stenosis is defined as a web-like, membranous concentric stenosis without damage to the cartilage and less than one centimeter in length. By comparison, complex stenoses are lesions more than one centimeter in length and/or with destruction of a cartilaginous structure or structures (27). In a single-center retrospective review, PTTS patients were found to have more complex stenoses, lower success rates with nonsurgical interventions, and lower rates of stent or tracheostomy tube removal (36). Recent data suggests a separate dichotomy into keloid and non-keloid lesions, either of which can occur in PITS or PTTS. PTTS keloid patients were noted to have earlier stenosis, poorer treatment outcomes, and increased recurrence when compared to PITS or non-keloid PTTS (37). It is well established complex lesions more require surgical intervention such as tracheal resection (27,36).
For simple stenosis, a therapeutic bronchoscopy is often the initial intervention, with therapies including thermo-ablative tissue destruction, dilation, and application of anti-inflammatory or antifibrotic agents. Mucosal-sparing radial incisions can be performed using either electrocautery knife/needle or LASER prior to dilation. Dilation can be done using the rigid bronchoscope barrel or continuous radial expansion balloons under direct visualization. In a single center study, the combination of radial incisions with an electrocautery knife with balloon dilation resulted in greater improvement in the degree of stenosis and decreased recurrence rates compared to balloon dilation alone (38). Cryospray has also demonstrated benefit in reducing symptoms and severity of airway stenosis in PITS and PTTS (39,40).
Endoscopic application of medications have been evaluated for benefit in the management of PITS and PTTS. Localized corticosteroid injection, to suppress inflammation and prevent further airway scarring, has been conjectured to prevent disease recurrence, but there has been little success with animal studies (41-44). There is some evidence of benefit for injection in the office setting, as well as some evidence for systemic steroids in those with recurrent disease to diminish need for repeated dilation or surgical management (45,46). Mitomycin C, a DNA-crosslinking agent, has antineoplastic and anti-fibroblastic properties and has also been theorized to prevent recurrence. Mitomycin C has demonstrated efficacy in animal studies (47), but use in humans has demonstrated to mixed results, with some signal of potential benefit of increasing interval to repeat procedures (48-50).
For complex stenosis, bronchoscopic intervention can be utilized to stabilize patients with critical narrowing or severe symptoms, but definitive therapy should be discussed within a multi-disciplinary team given the high risk of recurrence (26,27). Definitive surgical management is resection with primary tracheal anastomosis and should be considered based on time to recurrence or for those without symptom or disease control after nonsurgical intervention. For non-surgical candidates, tube stents, tracheostomy tube, or Montgomery T-tubes can be placed for safety or symptom control. Based on limited case series, placement of silicone stents within the first 6 months of presentation have been showed to allow for remodeling and eventually stent removal (51). Data suggests silicone stenting has an acceptable safety and tolerance (22,52). There is limited data for other stents, with one case series of fully covered metal stents in a mixed population of nonoperative tracheal stenosis patients demonstrating high complication rates, including need for stent removal (53).
The coronavirus disease 2019 (COVID-19) pandemic resulted in record numbers of patients requiring intubation and mechanical ventilation with higher mean duration of ventilation days, and increased rates of reintubation and tracheostomy reported at some centers (54,55). The aftermath of the pandemic is predicted by experts to increase the overall incidence of PITS and PTTS, but population data has yet to be published (56). Initial proposed management strategies are comparable to non-COVID-19 patients, include bronchoscopic and surgical interventions and initial case series suggest good success rates (57,58).
Case 1
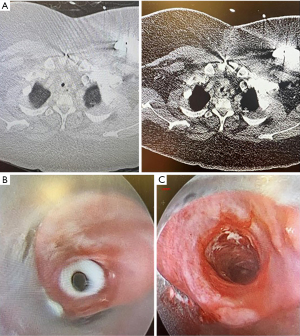
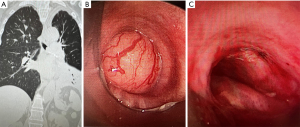
A 30-year-old morbidly obese female with prior COVID-19 infection requiring prolonged mechanical ventilation and complicated by cardiomyopathy requiring left ventricular assist device (LVAD) placement, presented two months post tracheostomy decannulation with inspiratory stridor. A CT showed mid-tracheal stenosis (Figure 2A) and bronchoscopy revealed concentric complex stenosis, four centimeters below the vocal cords and five centimeters above the main carina. The stenotic segment was two and a half centimeters in length and the lumen diameter was 4–5 millimeters (Figure 2B). Combined rigid and flexible bronchoscopy was used for initial radial cuts using rigid scissors and an electrocautery knife, followed by balloon and rigid barrel dilation to 10 mm diameter (Figure 2C). The patient had subjective improvement after the procedure. However, she re-presented within one month following initial dilation with similar symptoms and repeat dilation was done in similar manner. Thoracic surgery evaluated the patient at the time of the second dilation and she was deemed a high-risk surgical candidate for tracheal resection given morbid obesity and LVAD-dependence; surgery was deferred. Given the complex nature of her stenosis, along with rapid recurrence, decision was made to proceed with tracheal silicone stent placement (not shown). Patient had good control of respiratory symptoms in the 3 months post-stent placement and required no further airway procedures.
Idiopathic subglottic stenosis
Idiopathic subglottic stenosis most often effects females, typically presenting in the fourth or fifth decade of life (59). Surgical specimens have suggested a potential role for hormone receptor imbalance; recent genomic data suggests a role for several genes involved with epithelial-mesenchymal transition (60,61). There is also data supporting genetic risks, with reports of familial clustering (62). Delays in diagnosis are common, with more than 30% of patients in one case series diagnosed with asthma and another with average time to diagnosis of greater than 18 months (63,64). It is a diagnosis of exclusion and other pathologies, such as systemic autoimmune diseases and infections, must be ruled out. Idiopathic subglottic stenosis is usually a short length and circumferential lesion involving the cricoid ring, with histology showing keloid-like fibrosis and epithelial squamous metaplasia with sparing of the cartilage (59).
Like PITS and PTTS, a simple stenosis is often treated endoscopically with radial incision and dilation, but recurrent or complex disease is more challenging to manage. Both otolaryngologists and pulmonologists have published studies of different endoscopic modalities leading to successful management. A single center study of 132 patients showed successful bronchoscopic treatment in 100% of simple stenoses, but only 69.8% in complex disease (65). A separate single center study supported utilizing endoscopic laryngotracheoplasty via suspension laryngoscopy with multiple modalities including CO2 laser dissection, steroid injection, and balloon dilation to increase success. This study of 54 patients revealed an overall 5-year success rate of 87.5% in a population of both simple and complex stenosis, in which 78% of patients were managed only endoscopically (66). The use of radiofrequency coblation, utilizing bipolar energy to ablate and coagulate soft tissues at low temperatures via suspension laryngoscopy has also been described as successful (67,68). Recurrent disease is common, with one study showing a mean of 3.8 dilations required, albeit separated by long average inter-dilation interval of 90.7 weeks (69). There is also some conflicting data regarding different treatment modalities, including one recent study demonstrating CO2 laser treatment being associated with an increased risk of recurrence over balloon dilation alone, a difference from prior studies (18). Upcoming multicenter trials are planned to attempt to answer this question more definitively (70).
For non-procedural management, while idiopathic subglottic stenosis is often associated with gastroesophageal reflux, there is mixed data on the utility of proton-pump inhibitors, as well as conflicting data around the support of Bactrim, other antibiotics, or inhaled corticosteroids (64,71,72). Studies of steroid injection, known to be helpful in keloids, has had mixed results in studies (73,74). Mitomycin C has not been studied in idiopathic subglottic stenosis populations alone, but in mixed populations with mixed results (20,75)
Patients with disease recurrence or disease nonamenable to endoscopic management subglottic stenosis should be evaluated by an ear, nose, and throat (ENT) or thoracic surgeon for resection (66). If surgery is not an option, stenting, tracheostomy tube, or silicone T-tube placement can be used in cases with critical narrowing or rapid recurrence (52).
Systemic inflammatory diseases with airway manifestations
Many systemic inflammatory disorders can manifest with airway complications, including sarcoidosis, amyloidosis, relapsing polychondritis, granulomatosis with polyangiitis (GPA), inflammatory bowel disease, and other autoimmune connective tissue diseases (76). Treatment of the underlying systemic disorder in these cases often results in control of the airway disorder. However, when inflammatory airway obstruction has already occurred, reversal of the obstruction with systemic therapy is variable, and bronchoscopic intervention may be required to alleviate symptoms.
Granulomatous inflammation of the submucosal vasculature and airway mucosa in GPA can result in subglottic stenosis, thought to occur in up to 20% of GPA patients (77,78). More distal bronchial stenosis is also common, associated with higher recurrence than subglottic disease only, and can result in disabling multilevel stenosis (79). There is limited evidence for the best practice for airway stenosis management in patients with GPA. Radial incision with lasers or electrocautery; radiofrequency coblation; balloon dilation; and local steroid injection have all demonstrated efficacy in small studies (68,80-83). High dose systemic steroids and other systemic immunosuppressants such as cyclophosphamide and rituximab have been proven beneficial (79,84). Availability of effective systemic therapy, frequency of multi-level stenosis, and the risk of restenosis limits the role of surgery for these patients.
In contrast, benign relapsing polychondritis is characterized by inflammatory destruction of cartilage and sparing of the posterior membrane, with airway involvement occurring in up to 20–50% of patients and contributing to both morbidity and mortality (85-89). Initial obstruction due to cartilaginous edema is often followed by malacia from cartilaginous damage, which can lead to a combined fixed and dynamic obstruction. In a recent large case series, respiratory failure or infection from airway obstruction accounted for 65.4% of deaths (89). Airway stenosis in this population may be under-recognized, with up to 94% of patients having abnormal CT expiratory imaging in one case series (90). There is limited data on the management of airway involvement outside of systemic immunosuppression, though proximal airway malacia may necessitate placement of a tracheostomy to prevent death from airway collapse (91). A single center series demonstrated self-expanding metal stents (SEMS) improved dyspnea as well as facilitated weaning from mechanical ventilation (92).
In the common inflammatory disease sarcoidosis, airway involvement in any form is observed in approximately two-thirds of patients (93). Multiple pathophysiologies can result in obstruction, including extrinsic compression from lymphadenopathy, endobronchial granulomatous disease or scarring, or airway distortion from fibrosis. Airway obstruction can also be difficult to differentiate from sarcoid-induced hyperreactive airway disease, and treatment with systemic corticosteroids or other immunosuppressants improves obstruction from either cause (94). The deeper submucosal involvement of endobronchial disease may limit the utility of laser, electrocautery, topical mitomycin C, and cryotherapy, but each has been described as successful in small studies (95-99). Balloon dilatation and stenting can be utilized for patients without adequate response to systemic treatment (95,96,100).
Amyloidosis is a systemic disease involving extracellular amyloid fibril deposition and can affect many organ systems. Parenchymal nodules and pleural effusions are the most common pulmonary manifestations (101). Multifocal infiltration of amyloid plaques into airways is a rarer presentation, accounting for an estimated 1.1% of all amyloid cases, and not usually associated with detectable peripheral lymphoplasmacytic clonal proliferation (102). Bronchoscopy typically demonstrates irregular white deposits, often arising from the posterior wall, which may completely occlude the airway. Systemic therapy has been trialed with some success and there is no evidence to guide the choice of bronchoscopic therapy modalities, which can be necessary in central disease or more peripheral disease with near complete occlusion leading to infection or symptoms. Bronchoscopic treatment with laser or forceps resection, balloon dilation, as well as treatment with external radiation is described, but data is limited (103-108).
Case 2
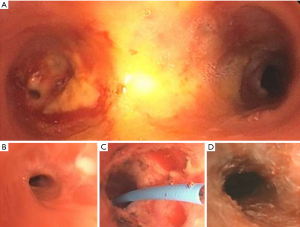
A 61-year-old female with GPA on systemic immunosuppression presented with shortness of breath and wheezing. She had required several bronchoscopies for dilation and stenting more than 2 years prior for left mainstem stenosis, but this had become quiescent after initiation of rituximab. CT revealed multi-level tracheobronchial stenosis with stenosis in the subglottic area as well as the left mainstem. On bronchoscopic exam, there was mild subglottic stenosis with estimated reduction of diameter to 30% and no intervention was done at that level. There was severe stenosis with a luminal diameter of <3 mm at the left mainstem (Figure 3A,3B), radial cuts using electrocautery knife followed by balloon dilation and steroid injection was performed (Figure 3C,3D). Recurrence of the stenosis required further dilations in the 18 months of follow-up, but patency was able to be maintained.
Infectious causes of central airway stenosis
Viral, bacteria, mycobacterial, and fungal infections can affect the airways with varying presentation and severity. Infectious obstruction can take the form of severe airway inflammation, endobronchial lesion(s), or airway stenosis. Treatment of underlying infection will often result in resolution of airway disease. However, some infections, such as tuberculosis (TB), can result in chronic, fibrotic airway stenosis, while others, like recurrent respiratory papillomatosis (RRP), have a recurrent and progressive nature.
RRP, caused by human papillomavirus (HPV) types 6 and 11, results in debilitating chronic disease in both adult and pediatric patients with a current estimated incidence of two cases per 100,000 adults (109). However, the overall incidence is declining in multiple first-world countries due to increased utilization of HPV vaccinations (110,111). Papillomas typically appear as white, polypoid lesions with smooth surfaces and can affect any part of tracheobronchial tree, from vocal cords down to and including the lung parenchyma, and there is potential for malignant transformation (112). Endoscopic removal of obstructing papillomas is the primary treatment modality, and recurrence rate approaches 100% as the HPV virus is thought to be dormant even in non-papillomatous epithelial cells (113,114). Both laryngologists’ and bronchoscopists’ skillsets are needed in these complex patients with laryngoscopy and microsurgery for supraglottic vocal cord, subglottic, and proximal tracheal papillomas, and flexible and rigid bronchoscopy for more distal tracheal and bronchial papillomas. Given the recurrence rates, procedural goals are noncurative, but focus on maintaining adequate voice quality and airway patency. Studies have shown success with lasers, including potassium titanyl phosphate (KTP), pulsed dye and CO2 lasers as well as microdebriders, rigid mechanical debridement, or cryotherapy (115,116). Tracheostomy placement is reserved for impending airway compromise, with consider of early decannulation given a risk of development or spreading of trachea papillomas (117). Though transmission is unproven, there is potential to aerosolize viral particles in procedures for RRP and personal protective equipment is paramount, with airborne precautions, including eye protection, a necessity for all entering or cleaning the room prior to air turnover (118).
Multiple medications have been studied in RRP and data is promising. Endoscopic intralesional injection of cidofovir, a cytosine analog, is well described with a systematic review of fourteen smaller studies showing an overall remission rate of 73.6% (119). In a case series of five patients treated with systemic bevacizumab, an antiangiogenic, all patients had a rapid and sustained response with a reduction in the number of bronchoscopic interventions required; there is also limited data for bevacizumab use intra-lesionally (119,120). Treatment with immunotherapy agents, including avelumab and bintrafusp alfa, has also demonstrated improvement in outcomes (121,122). There is now also evidence for use of HPV vaccination even after disease occurrence, with efficacy in decreasing the frequency of procedures (123,124).
TB most commonly involves the lobar bronchi, affecting up to 50% of patients with active TB and can persist even after antituberculosis treatment has been completed. Endobronchial TB is classified into one of seven subtypes: active caseating, edematous-hyperemic, fibro-stenotic, tumorous, granular, ulcerative, and nonspecific bronchitic (125). Success with balloon dilation, mitomycin-C application, CO2 and Nd:YAG laser therapy, cryotherapy, electrocautery, and stent placement have each been described (126-128). One recent retrospective analysis of stenosis in TB patients suggested both disease recurrence and development of malacia were increased in patients who underwent airway intervention, recommending patients diagnosed with TB early should be managed conservatively (129).
Fungal infections can present with airway involvement in both immunocompetent and immunocompromised hosts. Immunocompromised hosts are susceptible to invasive opportunistic fungal infections including mucormycosis, Fusarium infection and cryptococcal infections, while endemic fungal infections such as blastomycosis or coccidioidomycosis can present with airway disease even in patients with intact immune systems (130). Aspergillosis, the most common endobronchial fungal disease, is more common in immunocompromised hosts, but can occasionally occur in healthy individuals. There are four patterns identified for airway aspergillosis: pseudomembranous, ulcerative, obstructive, and invasive (131,132). Targeted antifungal antibiotics are the mainstay of treatment and multi-modality bronchoscopy has been described for both diagnosis and treatment (130).
Case 3
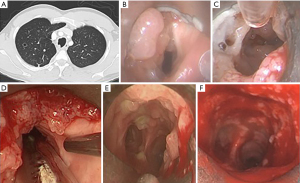
A 29-year-old male with a history of HPV infection since infancy presented with worsening dyspnea as well as vocal changes; he was previously managed with suspension laryngoscopy for papilloma removal from the vocal cords and proximal trachea once to twice a year. CT imaging revealed more distal polyps (Figure 4A). A joint procedure with a laryngologist and interventional pulmonologist was performed; suspension laryngoscopy revealed obstructing polyps both above and below the vocal cords that were removed with a microdebrider (Figure 4B-4D). Rigid bronchoscopy with mechanical debulking with the rigid barrel was then performed in the trachea and right and left mainstems with good result (Figure 4E,4F). Papillomas removed from the right mainstem revealed malignant transformation. He required a repeat procedure within 10 weeks, but after initiation of immunotherapy for his malignancy, all papilloma regrowth slowed, and procedure interval was able to be lengthened.
Central airway disease caused by benign tumors
Tracheobronchopathia osteochondroplastica (TO) is a rare disorder wherein multiple osseocartilaginous nodules are distributed over the cartilaginous rings of the tracheobronchial tree, with sparing of posterior membrane and the spaces between rings (133). These lesions are very firm, limiting biopsy and making effective dilation challenging. Fortunately, most TO patients are asymptomatic with lesions discovered incidentally, and can be managed conservatively with observation. TO can rarely cause severe tracheal stenosis requiring therapeutic bronchoscopy to maintain patency and limit symptoms. Use of vaporizing therapy, such as laser, combined with or without a rigid bronchoscopy mechanical coring technique has been described with good success, though data is limited (134,135).
Endobronchial hamartomas are benign lung malformations and can include a mixture of tissues including cartilage, fat, muscle and epithelium (136). Though more common in the pulmonary parenchyma, hamartomas are the most common benign airway tumor with 1.4% of cases presenting in an endobronchial location (137). Bronchoscopic treatment modalities include mechanical dissection, laser, cryotherapy, and argon plasma coagulation have been described with good result (138-140). Surgical resection should be reserved for those with symptoms from disease not amenable to bronchoscopic resection (138).
Many other less common benign tumors can occur in the airways, including chondroma, epitheliomas, lipomas, hemangiomas (141).
Case 4
A 65-year-old-female presented with recurrent left lower lobe pneumonias. A CT was performed and revealed a large left mainstem endobronchial tumor (Figure 5A), complicated by left lower lobe obstructive pneumonia. Rigid bronchoscopy revealed a large mid left mainstem tumor completely occluding the left mainstem; the surface of mass was vascular and glistening (Figure 5B). Given the predominant endobronchial component of the tumor with narrow pedunculated base, it was resected with electrocautery snare and then removed en bloc using cryo-adhesion with no residual tumor seen and restoration of 100% patency of the left lower lobe bronchus (Figure 5C). Histopathology was consistent with a pleomorphic adenoma, a benign tumor commonly originating in a salivary gland, but rarely described as arising from the airway. Follow up CT and bronchoscopy after 6 months showed no recurrence.
Dynamic airway obstruction
Excessive central airway collapse (ECAC) is disproportionate (>50%) narrowing of the airway lumen during all or part of expiration leading to symptoms of dyspnea, barking cough, mucus production, recurrent infections, and/or low quality of life. Excessive dynamic airway collapse (EDAC) and tracheobronchomalacia (TBM) are two separate pathologies within ECAC (142,143). EDAC is the excessive bowing the posterior membrane and TBM is the softening or loss of strength of the cartilaginous airway. EDAC and TBM can also co-exist. Spirometry can be suggestive of and expiratory CT is very sensitive for both EDAC and TBM components (144,145). Multiple co-pathologies, including COPD, asthma, chronic infections, gastroesophageal reflux disease (GERD), autoimmune disease, and obesity can contribute to ECAC and treatment of each should be medically optimized in symptomatic patients (143). The diagnosis of symptomatic ECAC should be suspected when usual treatment of other disease pathologies does not result in improvement.
Procedural management in ECAC is uniquely, compared to other causes of benign airway obstruction, distinctly divided into diagnostic and therapeutic maneuvers. Diagnostic bronchoscopy under minimal sedation is key to evaluate the severity of collapse and identify which airways are involved. Therapeutically, continuous positive airway pressure (CPAP) can be used as a pneumatic stent, and titration bronchoscopy to visual CPAP effect has been described as both a bridge to therapy and definitive management (143,146). In patients with a predominant central airway pattern and limited peripheral involvement, tracheal and bronchial stenting can visually and symptomatically improve ECAC. However, the long-term side effects of granulation tissue formation, stent migration, mucus plugging, and need for repeat procedures outweigh the benefits of definitive stenting in most patients with ECAC. Surgical tracheoplasty is an option in ECAC patients with symptomatic central disease. For potential surgical candidates, a role for stent trials to evaluate for symptomatic and physiologic benefit has been described (147,148). Both robotic and open surgical approaches have been described with good symptomatic success (149,150).
A word on airway stenting in non-transplant benign airway obstruction
Airway stenting is utilized in malignant airway obstruction to maintain luminal patency and provide structural support as a bridge to cancer-targeted therapy or for palliation. In benign airway obstruction, consideration of stenting must often account for a longer life expectancy, as well as lower morbidity of the disease process itself. Currently available airway stents include various sizes and shapes of both silicone stents and metallic stents; metallic stents are available as uncovered, partially covered, and fully covered (24). Patient specific customization via silicone 3D-printed stents, have also recently available and deserve further study (15,16,151,152). Metallic stents can be deployed using a combination of flexible or rigid bronchoscopy, airway guidewires, and fluoroscopy, while silicone stents require rigid bronchoscopy. Stent selection is based on length, diameter, and shape of the stenosis as well as stent material, deployment methods, and procedural skillset and tools available. Stent complications are common and include migration, mucus plugging, and granulation tissue formation with reported complication rates of up to 50%, often requiring permanent removal or removal and revision (22,53,153-155). Indeed, granulation tissue formation from uncovered metallic stents is a particular challenge and high complication rates and difficulty with removal were reported in the early 2000s (153,154). The U.S. FDA applied a black box warning on the use of metallic stents in central benign airway stenosis in 2005 (156). Additional covered or partially covered metallic stents have come to market since this warning. Studies of the use of covered metallic stents and silicone in benign airway stenosis suggest fewer complications (22,53,155). Our practice is to use airway stents as a last resort in benign disease, with anticipatory discussions with the patient, close monitoring post placement including planned repeat bronchoscopy, and anticipation and optimization for timely removal if possible.
Transplant airway complications
Airway complications of lung transplant include anastomotic necrosis and/or dehiscence, endobronchial infection, granulation tissue formation, fistula formation, anastomotic stenosis, stenosis at airways distal to anastomoses, and malacia. The reported incidence of airway complication post-transplant varies widely between 2% and 18% but has decreased over time (157-159). Time since transplant is associated with complication type, with dehiscence being most common in the immediate post operative period and stenosis later in the course.
Bronchial anastomoses are particularly susceptible to ischemia, due to the severing of the bronchial artery during transplant. The remaining perfusion via retrograde pulmonary arterial flow is restored intraoperative at time of pulmonary arterial anastomosis and revascularization occurs 2–4 weeks post-transplant, though a small study of perfusion scans in patients post-transplant demonstrated persistent distal airway hypoxemia even at 3 and 12 months (160). Risks factors for ischemia and airway complications have been identified and include the length of the donor bronchus, donor-recipient height mismatch, surgical techniques, donor ventilation time, and extracorporeal membrane oxygenation (ECMO) in the recipient (161-163).
In 2018, the International Society for Heart and Lung Transplant (ISHLT) published a consensus statement on airway complication including definitions, grading system, and recommended therapeutic interventions (164). The gradation system developed by the ISHLT accounts for the pathophysiologic change(s), the location, and the severity or extent of each abnormality. A summary of the therapeutic recommendations for complication type are noted in Table 2, with evaluation and treatment of infection also playing a critical role in management. Overall, combined data for all complications would suggest, similar to non-transplant benign disease, stenting in particular has a high complication rate and need for repeat procedures (165,166). It does have demonstrated efficacy in symptom management but no data on overall survival has yet been demonstrated (167).
Table 2
| Airway complication | ISHLT recommendation |
|---|---|
| Dehiscence | Partial airway thickness: conservative management with antibiotics and frequent surveillance bronchoscopy |
| Full thickness: placement of a covered or uncovered self-expanding metallic stent to induce granulation and healing prior to consideration of surgical repair | |
| Stenosis | Initial step: balloon dilation at regular intervals |
| If requiring >2 dilations/month with symptomatic benefit, stent placement | |
| Airway debridement if stenosis is due to granulation tissue or webbing | |
| Malacia | If critical ill and ventilatory support cannot be weaned, consider airway stenting |
| If not critically ill, consider noninvasive positive pressure prior to a stenting or surgical correction |
ISHLT, International Society for Heart and Lung Transplantation.
The management of the early complication anastomotic necrosis or dehiscence is driven by the severity and presence of complications such as abscess or fistula. Necrosis without dehiscence can be managed conservatively with frequent surveillance bronchoscopy to assess for progression or complications (164). Extensive necrosis or dehiscence can be treated with self-expanding metallic stent placement. Uncovered stents are favored to promote neo-epithelization are favored by some studies, while utilizing covered stents to seal airway defects by other (168,169). Silicone stents are generally avoided due to the force required for placement (170). Overall, the practice remains controversial due to high rates of complications. Salvage surgical maneuvers are challenging, and most case reports note the requirement for tissue grafting to reinforce the necrotic anastomosis (171-173).
Bronchial stenosis is the most common airway complication post transplantation and can occur anytime from months to years post-transplant. Airway stenosis is classified as central if occurring within 2 cm of the anastomosis and distal if greater than 2 cm from the anastomosis (164). The vanishing bronchus syndrome is stenosis of the bronchus intermedius distal to the right anastomosis and occurs in approximately 2% of right lung transplants. It is associated with increased morbidity and mortality with a mean survival of 25 months (174). Like non-transplant benign stenosis, if a stenosis is less than 50% of the normal lumen diameter and the patient is asymptomatic a watchful waiting approach is recommended; while if greater than 50%, bronchoscopic therapeutic intervention has to be considered. Balloon dilation alone is often the first treatment modality, but may need to be repeated to be effective, with an average of four balloon dilations to obtain good result in the largest case series (175). If balloon dilation is ineffective, limited data exists to support ablative therapies, stenting, brachytherapy, and topical therapy (176-181). Surgery is described by the ISHLT as the nonpreferred approach, but multiple surgical maneuvers have been described in case reports or series (182-184). The ISHLT highlight the importance of noting if malacia is resulting in airway narrowing and recommends treating malacia conservatively in the absence of critical illness, severe symptoms, or functional impairment (164).
Strengths and limitations
The range of subspecialists, procedures, surgeries, medications, and other therapies available to manage the varied pathologies making up benign airway obstruction are both a strength and weakness for these complex patients. The number of case series demonstrating high rates of success is heartening, but the frequent lack of multi-center or prospective data challenges interpretation. The delay to diagnosis in many patients certainly influences outcomes and conflicting data likely also represents practice preference, local expertise, and differences in patient populations. The investment of providers from multiple specialties and subspecialties widens the cumulative cognitive and technical skillset but can lead to fractures in ongoing progress in improving our understanding and management. The next years will be particularly revealing as more data becomes available from the challenging post-COVID 19 population and additional insight from electronic medical record (EMR) based artificial intelligence (AI) and genomics improves diagnostic acumen. The suggestion for a multi-disciplinary approach for benign airway stenosis should not just apply to patient management, but also for ongoing research. With the universal availability of electronic health records, virtual work platforms, and, soon, AI, there is no reason accurate and high-impact data, including prospective clinical trials, could not be forthcoming.
Conclusions
In summary, diagnosis and management of benign airway obstruction must include a high clinical suspicion, fastidious triage and stabilization, understanding and assessment of each patient’s pathology and physiology, and meticulous procedural planning. Treating providers must be realistic with each patient about expected outcomes from procedures, the need for frequent reassessment, and the potential for repeat procedures and referrals to other specialists. While the field of interventional bronchoscopy continues to expand in both availability and toolkit, we cannot underemphasize the role of a surgical subspecialists and a multidisciplinary approach to achieving patients’ goals safely, consistently, and for meaningful durations of time. We must remember evidence-based data in these diseases is often limited and personalizing the approach to management in a thoughtful manner is critical for success.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Jonathan Kurman and Bryan S. Benn) for the series “Diagnostic & Therapeutic Bronchoscopy” published in AME Medical Journal. The article has undergone external peer review.
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-103/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-103/coif). The series “Diagnostic & Therapeutic Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohan A, Shrestha P, Madan K, et al. A Prospective Outcome Assessment After Bronchoscopic Interventions for Malignant Central Airway Obstruction. J Bronchology Interv Pulmonol 2020;27:95-105. [Crossref] [PubMed]
- Myer CM 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 1994;103:319-23. [Crossref] [PubMed]
- Monnier P, Dikkers FG, Eckel H, et al. Preoperative assessment and classification of benign laryngotracheal stenosis: a consensus paper of the European Laryngological Society. Eur Arch Otorhinolaryngol 2015;272:2885-96. [Crossref] [PubMed]
- Freitag L, Ernst A, Unger M, et al. A proposed classification system of central airway stenosis. Eur Respir J 2007;30:7-12. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Mark EJ, Meng F, Kradin RL, et al. Idiopathic tracheal stenosis: a clinicopathologic study of 63 cases and comparison of the pathology with chondromalacia. Am J Surg Pathol 2008;32:1138-43. [Crossref] [PubMed]
- Noppen M, Meysman M, D’Haese J, et al. Interventional bronchoscopy: 5-year experience at the Academic Hospital of the Vrije Universiteit Brussel (AZ-VUB). Acta Clin Belg 1997;52:371-80. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Miller RD, Hyatt RE. Obstructing lesions of the larynx and trachea: clinical and physiologic characteristics. Mayo Clin Proc 1969;44:145-61. [PubMed]
- Kossyvaki V, Anagnostopoulos N, Kaltsakas G, et al. The Value of Dyspnea and Spirometry in Detecting Relapse of Benign Tracheal Stenosis. Respiration 2022;101:174-83. [Crossref] [PubMed]
- Grenier PA, Beigelman-Aubry C, Fétita C, et al. New frontiers in CT imaging of airway disease. Eur Radiol 2002;12:1022-44. [Crossref] [PubMed]
- Shiau M, Harkin TJ, Naidich DP. Imaging of the central airways with bronchoscopic correlation: pictorial essay. Clin Chest Med 2015;36:313-34. ix-x. [Crossref] [PubMed]
- Hoppe H, Dinkel HP, Walder B, et al. Grading airway stenosis down to the segmental level using virtual bronchoscopy. Chest 2004;125:704-11. [Crossref] [PubMed]
- Ferretti GR, Jankowski A, Perrin MA, et al. Multi-detector CT evaluation in patients suspected of tracheobronchomalacia: comparison of end-expiratory with dynamic expiratory volumetric acquisitions. Eur J Radiol 2008;68:340-6. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of complex airway stenoses using patient-specific 3D-engineered stents: a proof-of-concept study. Thorax 2019;74:810-3. [Crossref] [PubMed]
- Shan Q, Huang W, Shang M, et al. Customization of stent design for treating malignant airway stenosis with the aid of three-dimensional printing. Quant Imaging Med Surg 2021;11:1437-46. [Crossref] [PubMed]
- Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis. Management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest 1993;104:673-7. [Crossref] [PubMed]
- Ntouniadakis E, Sundh J, Magnuson A, et al. Balloon dilatation is superior to CO(2) laser excision in the treatment of subglottic stenosis. Eur Arch Otorhinolaryngol 2023;280:3303-11. [Crossref] [PubMed]
- Wierzbicka M, Tokarski M, Puszczewicz M, et al. The efficacy of submucosal corticosteroid injection and dilatation in subglottic stenosis of different aetiology. J Laryngol Otol 2016;130:674-9. [Crossref] [PubMed]
- Yung KC, Chang J, Courey MS. A randomized controlled trial of adjuvant mitomycin-c in endoscopic surgery for laryngotracheal stenosis. Laryngoscope 2020;130:706-11. [Crossref] [PubMed]
- Benn BS, Lum M, Krishna G. Bronchoscopic Treatment of Airway Obstructions With a Novel Electrosurgical Device. J Bronchology Interv Pulmonol 2021;28:34-41. [Crossref] [PubMed]
- Chen DF, Chen Y, Zhong CH, et al. Long-term efficacy and safety of the Dumon stent for benign tracheal stenosis: a meta-analysis. J Thorac Dis 2021;13:82-91. [Crossref] [PubMed]
- Marchioni A, Andrisani D, Tonelli R, et al. Stenting versus balloon dilatation in patients with tracheal benign stenosis: The STROBE trial. Laryngoscope Investig Otolaryngol 2022;7:395-403. [Crossref] [PubMed]
- Guibert N, Saka H, Dutau H. Airway stenting: Technological advancements and its role in interventional pulmonology. Respirology 2020;25:953-62. [Crossref] [PubMed]
- Anantham D. Management principles of nonmalignant airway obstruction. In: Ernst A, Herth FJF. Editors. Principles and Practice of Interventional Pulmonology. 1st edition. New York, NY, USA: Springer-Verlag; 2013:269-84.
- Agrawal A, Baird BJ, Madariaga MLL, et al. Multi-disciplinary management of patients with benign airway strictures: A review. Respir Med 2021;187:106582. [Crossref] [PubMed]
- Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J 1999;13:888-93. [Crossref] [PubMed]
- Nouraei SA, Ma E, Patel A, et al. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol 2007;32:411-2. [Crossref] [PubMed]
- Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis: Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-93. [Crossref] [PubMed]
- Zias N, Chroneou A, Tabba MK, et al. Post tracheostomy and post intubation tracheal stenosis: report of 31 cases and review of the literature. BMC Pulm Med 2008;8:18. [Crossref] [PubMed]
- Nouraei SSR, Battson RM, Koury EF, et al. Adult Post-Intubation Laryngotracheal Stenosis: An Underestimated Complication of Intensive Care? J Intensive Care Soc 2009;10:229. [Crossref]
- Koshkareva Y, Gaughan JP, Soliman AM. Risk factors for adult laryngotracheal stenosis: a review of 74 cases. Ann Otol Rhinol Laryngol 2007;116:206-10. [Crossref] [PubMed]
- Kastanos N, Estopá Miró R, Marín Perez A, et al. Laryngotracheal injury due to endotracheal intubation: incidence, evolution, and predisposing factors. A prospective long-term study. Crit Care Med 1983;11:362-7. [Crossref] [PubMed]
- Tadié JM, Behm E, Lecuyer L, et al. Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med 2010;36:991-8. [Crossref] [PubMed]
- Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J (Clin Res Ed) 1984;288:965-8. [Crossref] [PubMed]
- Shin B, Kim K, Jeong BH, et al. Clinical significance of differentiating post-intubation and post-tracheostomy tracheal stenosis. Respirology 2017;22:513-20. [Crossref] [PubMed]
- Chang E, Wu L, Masters J, et al. Iatrogenic subglottic tracheal stenosis after tracheostomy and endotracheal intubation: A cohort observational study of more severity in keloid phenotype. Acta Anaesthesiol Scand 2019;63:905-12. [Crossref] [PubMed]
- Bo L, Li C, Chen M, et al. Application of Electrocautery Needle Knife Combined with Balloon Dilatation versus Balloon Dilatation in the Treatment of Tracheal Fibrotic Scar Stenosis. Respiration 2018;95:182-7. [Crossref] [PubMed]
- Fernando HC, Dekeratry D, Downie G, et al. Feasibility of spray cryotherapy and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg 2011;40:1177-80. [Crossref] [PubMed]
- Bhora FY, Ayub A, Forleiter CM, et al. Treatment of Benign Tracheal Stenosis Using Endoluminal Spray Cryotherapy. JAMA Otolaryngol Head Neck Surg 2016;142:1082-7. [Crossref] [PubMed]
- Guven M, Turan F, Eyibilen A, et al. A comparison of the efficacy of 5-fluorouracil/triamcinolone, carnitine and dexamethasone therapy on wound healing in tracheal injury: potential for preventing tracheal stenosis? Eur Arch Otorhinolaryngol 2012;269:201-6. [Crossref] [PubMed]
- Supance JS. Antibiotics and steroids in the treatment of acquired subglottic stenosis. A canine model study. Ann Otol Rhinol Laryngol 1983;92:377-82. [Crossref] [PubMed]
- Doolin EJ, Tsuno K, Strande LF, et al. Pharmacologic inhibition of collagen in an experimental model of subglottic stenosis. Ann Otol Rhinol Laryngol 1998;107:275-9. [Crossref] [PubMed]
- Kil HK, Alberts MK, Liggitt HD, et al. Dexamethasone treatment does not ameliorate subglottic ischemic injury in rabbits. Chest 1997;111:1356-60. [Crossref] [PubMed]
- Bertelsen C, Shoffel-Havakuk H, O’Dell K, et al. Serial In-Office Intralesional Steroid Injections in Airway Stenosis. JAMA Otolaryngol Head Neck Surg 2018;144:203-10. [Crossref] [PubMed]
- Shadmehr MB, Abbasidezfouli A, Farzanegan R, et al. The Role of Systemic Steroids in Postintubation Tracheal Stenosis: A Randomized Clinical Trial. Ann Thorac Surg 2017;103:246-53. [Crossref] [PubMed]
- Eliashar R, Eliachar I, Esclamado R, et al. Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope 1999;109:1594-600. [Crossref] [PubMed]
- Madan K, Agarwal R, Aggarwal AN, et al. Utility of rigid bronchoscopic dilatation and mitomycin C application in the management of postintubation tracheal stenosis: case series and systematic review of literature. J Bronchology Interv Pulmonol 2012;19:304-10. [Crossref] [PubMed]
- Rahbar R, Shapshay SM, Healy GB. Mitomycin: effects on laryngeal and tracheal stenosis, benefits, and complications. Ann Otol Rhinol Laryngol 2001;110:1-6. [Crossref] [PubMed]
- Reichert LK, Zhao AS, Galati LT, et al. The Efficacy of Mitomycin C in the Treatment of Laryngotracheal Stenosis: Results and Experiences with a Difficult Disease Entity. ORL J Otorhinolaryngol Relat Spec 2015;77:351-8. [Crossref] [PubMed]
- Lim SY, Kim H, Jeon K, et al. Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J 2012;53:565-70. [Crossref] [PubMed]
- Puma F, Ragusa M, Avenia N, et al. The role of silicone stents in the treatment of cicatricial tracheal stenoses. J Thorac Cardiovasc Surg 2000;120:1064-9. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Meng L, Qiu H, Wan L, et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology 2020;132:1317-32. [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Piazza C, Filauro M, Dikkers FG, et al. Long-term intubation and high rate of tracheostomy in COVID-19 patients might determine an unprecedented increase of airway stenoses: a call to action from the European Laryngological Society. Eur Arch Otorhinolaryngol 2021;278:1-7. [Crossref] [PubMed]
- Thong G, Lorenz H, Sandhu GS, et al. Emergency presentation of iatrogenic airway stenosis following intubation in a patient with COVID-19 and its management. BMJ Case Rep 2020;13:e238508. [Crossref] [PubMed]
- Piazza C, Lancini D, Filauro M, et al. Post-COVID-19 airway stenosis treated by tracheal resection and anastomosis: a bicentric experience. Acta Otorhinolaryngol Ital 2022;42:99-105. [Crossref] [PubMed]
- Aravena C, Almeida FA, Mukhopadhyay S, et al. Idiopathic subglottic stenosis: a review. J Thorac Dis 2020;12:1100-11. [Crossref] [PubMed]
- Fiz I, Bittar Z, Piazza C, et al. Hormone receptors analysis in idiopathic progressive subglottic stenosis. Laryngoscope 2018;128:E72-7. [Crossref] [PubMed]
- Zhang C, Wang S, Casal Moura M, et al. RNA Sequencing of Idiopathic Subglottic Stenosis Tissues Uncovers Putative Profibrotic Mechanisms and Identifies a Prognostic Biomarker. Am J Pathol 2022;192:1506-30. [Crossref] [PubMed]
- Drake VE, Gelbard A, Sobriera N, et al. Familial Aggregation in Idiopathic Subglottic Stenosis. Otolaryngol Head Neck Surg 2020;163:1011-7. [Crossref] [PubMed]
- Gnagi SH, Howard BE, Anderson C, et al. Idiopathic Subglottic and Tracheal Stenosis: A Survey of the Patient Experience. Ann Otol Rhinol Laryngol 2015;124:734-9. [Crossref] [PubMed]
- Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope 2014;124:498-503. [Crossref] [PubMed]
- Dalar L, Karasulu L, Abul Y, et al. Bronchoscopic Treatment in the Management of Benign Tracheal Stenosis: Choices for Simple and Complex Tracheal Stenosis. Ann Thorac Surg 2016;101:1310-7. [Crossref] [PubMed]
- Nouraei SA, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope 2013;123:2474-84. [Crossref] [PubMed]
- Chan CL, Frauenfelder CA, Foreman A, et al. Surgical management of airway stenosis by radiofrequency coblation. J Laryngol Otol 2015;129:S21-6. [Crossref] [PubMed]
- Hseu AF, Benninger MS, Haffey TM, et al. Subglottic stenosis: a ten-year review of treatment outcomes. Laryngoscope 2014;124:736-41. [Crossref] [PubMed]
- Shabani S, Hoffman MR, Brand WT, et al. Endoscopic Management of Idiopathic Subglottic Stenosis. Ann Otol Rhinol Laryngol 2017;126:96-102. [Crossref] [PubMed]
- Soumagne T, Guibert N, Atallah I, et al. Dilation versus laser resection in subglottic stenosis: protocol for a prospective international multicentre randomised controlled trial (AERATE trial). BMJ Open 2022;12:e053730. [Crossref] [PubMed]
- Menapace DC, Ekbom DC, Larson DP, et al. Evaluating the Association of Clinical Factors With Symptomatic Recurrence of Idiopathic Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg 2019;145:524-9. [Crossref] [PubMed]
- Hoffman MR, Patro A, Huang LC, et al. Impact of Adjuvant Medical Therapies on Surgical Outcomes in Idiopathic Subglottic Stenosis. Laryngoscope 2021;131:E2880-6. [Crossref] [PubMed]
- Franco RA Jr, Husain I, Reder L, et al. Awake serial intralesional steroid injections without surgery as a novel targeted treatment for idiopathic subglottic stenosis. Laryngoscope 2018;128:610-7. [Crossref] [PubMed]
- Hoffman MR, Patro A, Huang LC, et al. Impact of Serial Intralesional Steroid Injections on Idiopathic Subglottic Stenosis. Laryngoscope 2023;133:2255-63. [Crossref] [PubMed]
- Feinstein AJ, Goel A, Raghavan G, et al. Endoscopic Management of Subglottic Stenosis. JAMA Otolaryngol Head Neck Surg 2017;143:500-5. [Crossref] [PubMed]
- Catano J, Uzunhan Y, Paule R, et al. Presentation, Diagnosis, and Management of Subglottic and Tracheal Stenosis During Systemic Inflammatory Diseases. Chest 2022;161:257-65. [Crossref] [PubMed]
- Martinez Del Pero M, Rasmussen N, Chaudhry A, et al. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur Arch Otorhinolaryngol 2013;270:345-54. [Crossref] [PubMed]
- Quinn KA, Gelbard A, Sibley C, et al. Subglottic stenosis and endobronchial disease in granulomatosis with polyangiitis. Rheumatology (Oxford) 2019;58:2203-11. [Crossref] [PubMed]
- Terrier B, Dechartres A, Girard C, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicentre experience. Rheumatology (Oxford) 2015;54:1852-7. [Crossref] [PubMed]
- Martinez Del Pero M, Jayne D, Chaudhry A, et al. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observational study. JAMA Otolaryngol Head Neck Surg 2014;140:1038-44. [Crossref] [PubMed]
- Nouraei SA, Obholzer R, Ind PW, et al. Results of endoscopic surgery and intralesional steroid therapy for airway compromise due to tracheobronchial Wegener’s granulomatosis. Thorax 2008;63:49-52. [Crossref] [PubMed]
- Wolter NE, Ooi EH, Witterick IJ. Intralesional corticosteroid injection and dilatation provides effective management of subglottic stenosis in Wegener’s granulomatosis. Laryngoscope 2010;120:2452-5. [Crossref] [PubMed]
- Hoffman GS, Thomas-Golbanov CK, Chan J, et al. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003;30:1017-21. [PubMed]
- Girard C, Charles P, Terrier B, et al. Tracheobronchial Stenoses in Granulomatosis With Polyangiitis (Wegener’s): A Report on 26 Cases. Medicine (Baltimore) 2015;94:e1088. [Crossref] [PubMed]
- Trentham DE, Le CH. Relapsing polychondritis. Ann Intern Med 1998;129:114-22. [Crossref] [PubMed]
- Chen N, Zheng Y. Characteristics and Clinical Outcomes of 295 Patients With Relapsing Polychondritis. J Rheumatol 2021;48:1876-82. [Crossref] [PubMed]
- Gergely P Jr, Poór G. Relapsing polychondritis. Best Pract Res Clin Rheumatol 2004;18:723-38. [PubMed]
- Ernst A, Rafeq S, Boiselle P, et al. Relapsing polychondritis and airway involvement. Chest 2009;135:1024-30. [Crossref] [PubMed]
- Lin DF, Yang WQ, Zhang PP, et al. Clinical and prognostic characteristics of 158 cases of relapsing polychondritis in China and review of the literature. Rheumatol Int 2016;36:1003-9. [PubMed]
- Lee KS, Ernst A, Trentham DE, et al. Relapsing polychondritis: prevalence of expiratory CT airway abnormalities. Radiology 2006;240:565-73. [PubMed]
- Hong G, Kim H. Clinical characteristics and treatment outcomes of patients with relapsing polychondritis with airway involvement. Clin Rheumatol 2013;32:1329-35. [PubMed]
- Sarodia BD, Dasgupta A, Mehta AC. Management of airway manifestations of relapsing polychondritis: case reports and review of literature. Chest 1999;116:1669-75. [PubMed]
- Polychronopoulos VS, Prakash UBS. Airway involvement in sarcoidosis. Chest 2009;136:1371-80. [Crossref] [PubMed]
- Lavergne F, Clerici C, Sadoun D, et al. Airway obstruction in bronchial sarcoidosis: outcome with treatment. Chest 1999;116:1194-9. [Crossref] [PubMed]
- Chapman JT, Mehta AC. Bronchoscopy in sarcoidosis: diagnostic and therapeutic interventions. Curr Opin Pulm Med 2003;9:402-7. [PubMed]
- Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest 2005;127:472-81. [Crossref] [PubMed]
- Fouty BW, Pomeranz M, Thigpen TP, et al. Dilatation of bronchial stenoses due to sarcoidosis using a flexible fiberoptic bronchoscope. Chest 1994;106:677-80. [Crossref] [PubMed]
- Teo F, Anantham D, Feller-Kopman D, et al. Bronchoscopic management of sarcoidosis related bronchial stenosis with adjunctive topical mitomycin C. Ann Thorac Surg 2010;89:2005-7. [PubMed]
- Carlin BW, Harrell JH 2nd, Moser KM. The treatment of endobronchial stenosis using balloon catheter dilatation. Chest 1988;93:1148-51. [Crossref] [PubMed]
- Fruchter O, Abed El Raouf B, Rosengarten D, et al. Long-term Outcome of Short Metallic Stents for Lobar Airway Stenosis. J Bronchology Interv Pulmonol 2017;24:211-5. [Crossref] [PubMed]
- Picken MM. The Pathology of Amyloidosis in Classification: A Review. Acta Haematol 2020;143:322-34. [Crossref] [PubMed]
- O’Regan A, Fenlon HM, Beamis JF Jr, et al. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine (Baltimore) 2000;79:69-79. [Crossref] [PubMed]
- Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc 2000;75:1148-52. [Crossref] [PubMed]
- Hui AN, Koss MN, Hochholzer L, et al. Amyloidosis presenting in the lower respiratory tract. Clinicopathologic, radiologic, immunohistochemical, and histochemical studies on 48 cases. Arch Pathol Lab Med 1986;110:212-8. [PubMed]
- Lu X, He B, Wang G, et al. Bronchoscopic Diagnosis and Treatment of Primary Tracheobronchial Amyloidosis: A Retrospective Analysis from China. Biomed Res Int 2017;2017:3425812. [PubMed]
- Maiwand MO, Nath AR, Kamath BSK. Cryosurgery in the Treatment of Tracheobronchial Amyloidosis. Journal of Bronchology 2001;8:95-7.
- Neben-Wittich MA, Foote RL, Kalra S. External beam radiation therapy for tracheobronchial amyloidosis. Chest 2007;132:262-7. [Crossref] [PubMed]
- Ren S, Ren G. External beam radiation therapy is safe and effective in treating primary pulmonary amyloidosis. Respir Med 2012;106:1063-9. [Crossref] [PubMed]
- Carifi M, Napolitano D, Morandi M, et al. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag 2015;11:731-8. [PubMed]
- Novakovic D, Cheng ATL, Zurynski Y, et al. A Prospective Study of the Incidence of Juvenile-Onset Recurrent Respiratory Papillomatosis After Implementation of a National HPV Vaccination Program. J Infect Dis 2018;217:208-12. [PubMed]
- Meites E, Stone L, Amiling R, et al. Significant Declines in Juvenile-onset Recurrent Respiratory Papillomatosis Following Human Papillomavirus (HPV) Vaccine Introduction in the United States. Clin Infect Dis 2021;73:885-90. [Crossref] [PubMed]
- Fortes HR, von Ranke FM, Escuissato DL, et al. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med 2017;126:116-21. [Crossref] [PubMed]
- Wilcox LJ, Hull BP, Baldassari CM, et al. Diagnosis and management of recurrent respiratory papillomatosis. Pediatr Infect Dis J 2014;33:1283-4. [Crossref] [PubMed]
- Ivancic R, Iqbal H, deSilva B, et al. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol 2018;3:22-34. [Crossref] [PubMed]
- Xie X, Young J, Kost K, et al. KTP 532 nm laser for laryngeal lesions. A systematic review. J Voice 2013;27:245-9. [Crossref] [PubMed]
- Schraff S, Derkay CS, Burke B, et al. American Society of Pediatric Otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg 2004;130:1039-42. [Crossref] [PubMed]
- Labedz G, Scatolini ML, Ruvinsky S, et al. Factors Related to Extralaryngeal Spread in Juvenile Recurrent Respiratory Papillomatosis. Laryngoscope 2021;131:1652-6. [Crossref] [PubMed]
- Fox-Lewis A, Allum C, Vokes D, et al. Human papillomavirus and surgical smoke: a systematic review. Occup Environ Med 2020;77:809-17. [Crossref] [PubMed]
- Drejet S, Halum S, Brigger M, et al. A Systematic Review. Otolaryngol Head Neck Surg 2017;156:435-41. [Crossref] [PubMed]
- Mohr M, Schliemann C, Biermann C, et al. Rapid response to systemic bevacizumab therapy in recurrent respiratory papillomatosis. Oncol Lett 2014;8:1912-8. [Crossref] [PubMed]
- Bai K, Norberg SM, Sievers C, et al. Durable response in a patient with recurrent respiratory papillomatosis treated with immune checkpoint blockade. Head Neck 2022;44:E31-7. [Crossref] [PubMed]
- Robbins Y, Friedman J, Clavijo PE, et al. Dual PD-L1 and TGF-b blockade in patients with recurrent respiratory papillomatosis. J Immunother Cancer 2021;9:e003113. [Crossref] [PubMed]
- Goon P, Sauzet O, Schuermann M, et al. Recurrent Respiratory Papillomatosis (RRP)-Meta-analyses on the use of the HPV vaccine as adjuvant therapy. NPJ Vaccines 2023;8:49. [Crossref] [PubMed]
- Ponduri A, Azmy MC, Axler E, et al. The Efficacy of Human Papillomavirus Vaccination as an Adjuvant Therapy in Recurrent Respiratory Papillomatosis. Laryngoscope 2023;133:2046-54. [Crossref] [PubMed]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [Crossref] [PubMed]
- Mondoni M, Repossi A, Carlucci P, et al. Bronchoscopic techniques in the management of patients with tuberculosis. Int J Infect Dis 2017;64:27-37. [Crossref] [PubMed]
- Ryu YJ, Kim H, Yu CM, et al. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur Respir J 2006;28:1029-35. [Crossref] [PubMed]
- Lim SY, Park HK, Jeon K, et al. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011;16:959-64. [Crossref] [PubMed]
- Lee KCH, Tan S, Goh JK, et al. Long-term outcomes of tracheobronchial stenosis due to tuberculosis (TSTB) in symptomatic patients: airway intervention vs. conservative management. J Thorac Dis 2020;12:3640-50. [Crossref] [PubMed]
- Keshishyan S, DeLorenzo L, Hammoud K, et al. Infections causing central airway obstruction: role of bronchoscopy in diagnosis and management. J Thorac Dis 2017;9:1707-24. [Crossref] [PubMed]
- Cadena J, Thompson GR 3rd, Patterson TF. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect Dis Clin North Am 2021;35:415-34. [Crossref] [PubMed]
- Denning DW. Invasive aspergillosis. Clin Infect Dis 1998;26:781-803; quiz 804-5. [Crossref] [PubMed]
- Cho HK, Jeong BH, Kim H. Clinical course of tracheobronchopathia osteochondroplastica. J Thorac Dis 2020;12:5571-9. [Crossref] [PubMed]
- Leske V, Lazor R, Coetmeur D, et al. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine (Baltimore) 2001;80:378-90. [Crossref] [PubMed]
- Jabbardarjani HR, Radpey B, Kharabian S, et al. Tracheobronchopathia osteochondroplastica: presentation of ten cases and review of the literature. Lung 2008;186:293-7. [Crossref] [PubMed]
- Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleural Tumors. 3rd edition. Heidelberg: Springer Berlin, 2012.
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996;71:14-20. [Crossref] [PubMed]
- Abdel Hady SM, Elbastawisy SE, Hassaballa AS, et al. Is surgical resection superior to bronchoscopic resection in patients with symptomatic endobronchial hamartoma? Interact Cardiovasc Thorac Surg 2017;24:778-82. [Crossref] [PubMed]
- Kim SA, Um SW, Song JU, et al. Bronchoscopic features and bronchoscopic intervention for endobronchial hamartoma. Respirology 2010;15:150-4. [Crossref] [PubMed]
- Sahin AA, Aydiner A, Kalyoncu F, et al. Endobronchial hamartoma removed by rigid bronchoscope. Eur Respir J 1989;2:479-80. [Crossref] [PubMed]
- Insler JE, Seder CW, Furlan K, et al. Benign Endobronchial Tumors: A Clinicopathologic Review. Front Surg 2021;8:644656. [Crossref] [PubMed]
- Hammond K, Ghori UK, Musani AI. Tracheobronchomalacia and Excessive Dynamic Airway Collapse. Clin Chest Med 2018;39:223-8. [Crossref] [PubMed]
- Murgu S, Colt H. Tracheobronchomalacia and excessive dynamic airway collapse. Clin Chest Med 2013;34:527-55. [Crossref] [PubMed]
- Boiselle PM, Michaud G, Roberts DH, et al. Dynamic expiratory tracheal collapse in COPD: correlation with clinical and physiologic parameters. Chest 2012;142:1539-44. [Crossref] [PubMed]
- Aslam A, De Luis Cardenas J, Morrison RJ, et al. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Current Concepts and Future Directions. Radiographics 2022;42:1012-27. [Crossref] [PubMed]
- Olley L, Steier J, Kaltsakas G. Nocturnal continuous positive airway pressure offers symptomatic benefit in excessive dynamic airway collapse despite normal sleep study. J Thorac Dis 2023;15:829-34. [Crossref] [PubMed]
- Kheir F, Majid A. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Medical and Surgical Treatment. Semin Respir Crit Care Med 2018;39:667-73. [Crossref] [PubMed]
- Parikh M, Wilson J, Majid A, et al. Airway stenting in excessive central airway collapse. J Vis Surg 2017;3:172. [Crossref] [PubMed]
- Wright CD, Mathisen DJ. Tracheobronchoplasty for tracheomalacia. Ann Cardiothorac Surg 2018;7:261-5. [Crossref] [PubMed]
- Lazzaro R, Inra ML. Tracheobronchoplasty: Indications and Best Approaches. Thorac Surg Clin 2023;33:141-7. [Crossref] [PubMed]
- Cheng GZ, Folch E, Wilson A, et al. 3D Printing and Personalized Airway Stents. Pulm Ther 2017;3:59-66. [Crossref]
- Gildea TR, Young BP, Machuzak MS. Application of 3D Printing for Patient-Specific Silicone Stents: 1-Year Follow-Up on 2 Patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Lunn W, Feller-Kopman D, Wahidi M, et al. Endoscopic removal of metallic airway stents. Chest 2005;127:2106-12. [Crossref] [PubMed]
- Colreavy MP, Walsh MA. Nitinol tracheobronchial stents: a word of caution. Laryngoscope 2000;110:1070. [Crossref] [PubMed]
- Noppen M, Stratakos G, D’Haese J, et al. Removal of covered self-expandable metallic airway stents in benign disorders: indications, technique, and outcomes. Chest 2005;127:482-7. [Crossref] [PubMed]
- FDA Public Health Notification: Complications from Metallic Tracheal Stents in Patients with Benign Airway Disorders. 2005.
- van Berkel V, Guthrie TJ, Puri V, et al. Impact of anastomotic techniques on airway complications after lung transplant. Ann Thorac Surg 2011;92:316-20; discussion 320-1. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg 2007;84:401-9, 409.e1-4.
- Dhillon GS, Zamora MR, Roos JE, et al. Lung transplant airway hypoxia: a diathesis to fibrosis? Am J Respir Crit Care Med 2010;182:230-6. [Crossref] [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8; discussion 298. [Crossref] [PubMed]
- Li G, Mankidy B, Liu Z, et al. A Closer Look at Risk Factors Associated with Airway Complications in Lung Transplantation. J Heart Lung Transplant 2021;40:S356. [Crossref]
- Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT Consensus Statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. J Heart Lung Transplant 2018;37:548-63. [Crossref] [PubMed]
- Ma KC, Li M, Haas AR, et al. Efficacy and safety of airway stenting to treat anastomotic complications after lung transplant: a cohort study. J Thorac Dis 2020;12:3539-48. [Crossref] [PubMed]
- Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. [Crossref] [PubMed]
- Wang Z, Zhao B, Deng M, et al. Utility and safety of airway stenting in airway stenosis after lung transplant: A systematic review. Front Med (Lausanne) 2023;10:1061447. [Crossref] [PubMed]
- Chhajed PN, Tamm M. Uncovered metallic stents for anastomotic dehiscence after lung transplantation. J Heart Lung Transplant 2005;24:1447-8. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Usuda K, Gildea TR, Pandya C, et al. Bronchial Dehiscence. Journal of Bronchology 2005;12:164-5. [Crossref]
- D’Andrilli A, Ibrahim M, Andreetti C, et al. Transdiaphragmatic harvesting of the omentum through thoracotomy for bronchial stump reinforcement. Ann Thorac Surg 2009;88:212-5. [Crossref] [PubMed]
- Deeb ME, Sterman DH, Shrager JB, et al. Bronchial anastomotic stricture caused by ossification of an intercostal muscle flap. Ann Thorac Surg 2001;71:1700-2. [Crossref] [PubMed]
- McGiffin D, Wille K, Young K, et al. Salvaging the dehisced lung transplant bronchial anastomosis with homograft aorta. Interact Cardiovasc Thorac Surg 2011;13:666-8. [Crossref] [PubMed]
- Machuzak M, Santacruz JF, Gildea T, et al. Airway complications after lung transplantation. Thorac Surg Clin 2015;25:55-75. [Crossref] [PubMed]
- De Gracia J, Culebras M, Alvarez A, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33. [Crossref] [PubMed]
- Sundset A, Lund MB, Hansen G, et al. Airway complications after lung transplantation: long-term outcome of silicone stenting. Respiration 2012;83:245-52. [Crossref] [PubMed]
- Kapoor BS, May B, Panu N, et al. Endobronchial stent placement for the management of airway complications after lung transplantation. J Vasc Interv Radiol 2007;18:629-32. [Crossref] [PubMed]
- Meyer A, Warszawski-Baumann A, Baumann R, et al. HDR brachytherapy: an option for preventing nonmalignant obstruction in patients after lung transplantation. Strahlenther Onkol 2012;188:1085-90. [Crossref] [PubMed]
- Halkos ME, Godette KD, Lawrence EC, et al. High dose rate brachytherapy in the management of lung transplant airway stenosis. Ann Thorac Surg 2003;76:381-4. [Crossref] [PubMed]
- Cosano-Povedano J, Muñoz-Cabrera L, Jurado-Gámez B, et al. Topical Mitomycin C for Recurrent Bronchial Stenosis After Lung Transplantation: A Report of 2 Cases. Journal of Bronchology 2008;15:281-3. [Crossref]
- Mahajan AK, Folch E, Khandhar SJ, et al. The Diagnosis and Management of Airway Complications Following Lung Transplantation. Chest 2017;152:627-38. [Crossref] [PubMed]
- Marulli G, Loy M, Rizzardi G, et al. Surgical treatment of posttransplant bronchial stenoses: case reports. Transplant Proc 2007;39:1973-5. [Crossref] [PubMed]
- Camargo Jde J, Camargo SM, Machuca TN, et al. Surgical maneuvers for the management of bronchial complications in lung transplantation. Eur J Cardiothorac Surg 2008;34:1206-9. [Crossref] [PubMed]
- Paulson EC, Singhal S, Kucharczuk JC, et al. Bronchial sleeve resection for posttransplant stricture. Ann Thorac Surg 2003;76:2075-6. [Crossref] [PubMed]
Cite this article as: Hattab Y, de Salmeron SP, Malsin E. Benign airway obstruction: a clinical practice review of causes and managements principles. AME Med J 2024;9:4.

