
Complete pathological response of pelvic Ewing sarcoma in adult female—a case report
Highlight box
Key findings
• This report describes a rare case of extraosseous pelvic Ewing sarcoma which demonstrated a complete pathologic response after 8 cycles of vincristine, adriamycin, clophosphamide alternating with ifosfamide and etoposide (VAC-IE) chemotherapy.
What is known and what is new?
• As pelvic extraskeletal Ewing sarcoma is rare, there is very little data to guide a standard of care treatment protocol. Therefore, the treatment of osseous and extraosseous Ewing sarcoma are typically similar.
• This case report describes how VAC-IE chemotherapy can be an effective treatment for extraosseous pelvic Ewing sarcoma in adults.
What is the implication, and what should change now?
• For Ewing sarcoma, alternating vincristine sulfate, doxorubicin hydrochloride (adriamycin), and cyclophosphamide, followed by ifosfamide and etoposide phosphate is a well-accepted chemotherapy regimen.
Introduction
Background
Ewing sarcoma is a highly aggressive malignancy marked by high rates of progression after treatment and metastasis, diagnosed by immunofluorescence revealing an EWSR1-FLI1 fusion (1). Most Ewing sarcoma cases are of skeletal origin. However, a small number of cases are extraosseous. Pelvic Ewing sarcoma has been associated with significantly worse prognosis than Ewing sarcomas in other areas (2).
Rationale and knowledge gap
There have been a small number of cohort studies that have evaluated the response of pelvic Ewing sarcoma response to preoperative chemotherapy, such as this study following 10 patients with Ewing sarcoma of the pelvis (2). While this study and studies like it give evidence to pelvic Ewing sarcoma being treatable, there is a lack of standardization of treatment regimens. However, there is a lack of understanding and consensus in the treatment of this condition when present in adults.
Objective
There have been several reports focusing on Ewing sarcoma outcomes. However, most of these case reports have focused on metastatic progression of disease (1). This case report highlights a unique outcome in a patient with successful resection of a locally advanced pelvic Ewing sarcoma after complete response to neoadjuvant chemotherapy. We present this case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-23-36/rc).
Case presentation
In September 2021, A 41-year-old female presented with right flank pain. The patient had no significant past medical history. Prior to this presentation, the patient had very little interaction with the health care system. The patient underwent an ultrasound of the abdomen and pelvis which was found to be non-diagnostic. Over 2 months, the pain continued to worsen and began to radiate to the pelvis. After presenting to the emergency department in November 2021, computerized tomography (CT) scan was performed, which demonstrated a large right-sided pelvic mass (Figure 1). Initial evaluation of the mass was presumed to be emanating from the uterus with the impression that this was potentially uterine fibroid. Given the symptomatology, the patient underwent evaluation by gynecology and subsequent surgery for the presumed fibroid tumor. The case ultimately was converted to an open procedure due to difficulty. However, the pelvic mass was found to be unresectable due to encasement of the right ureter. A biopsy was performed intraoperatively, and the patient only underwent total abdominal hysterectomy and bilateral salpingectomy. The patient recovered from surgery uneventfully.
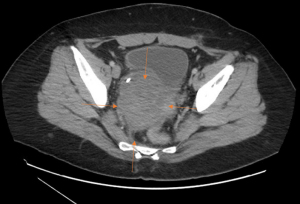
The intraoperative biopsy returned as extraosseous Ewing sarcoma (EES), positive for EWSR1-FLI1 fusion on immunohistochemistry (1). The patient was then referred to medical oncology. In December 2021, a staging positron emission tomography (PET) scan revealed a 9.8×8.5 cm mass in the right lateral wall of the uterus in the right hemipelvis. Magnetic resonance imaging (MRI) of right pelvic mass measured 8.9×10.7×9.6 cm and exhibited encasement of the right ureter with bladder wall involvement. Pathology was re-reviewed at our center with the operative pathology and immunofluorescence again revealed an EWSR1-FLI1 fusion, which is diagnostic for Ewing sarcoma (1). The patient was diagnosed with a clinical stage II, cT3, cN0, cM0 pelvic Ewing sarcoma and was presented at our multidisciplinary sarcoma tumor board with the recommendation to proceed with systemic chemotherapy utilizing vincristine, adriamycin, clophosphamide alternating with ifosfamide and etoposide (VAC-IE). The patient received VAC on day 1 of each cycle, then received IE for 5 days, with a 2-week interval placed between each VAC and IE treatment. Mesna was given alongside cyclophosphamide and ifosfamide to prevent hemorrhagic cystitis. The patient’s original dosing is listed in Table 1. Despite some tolerability concerns the patient was able to complete 8 cycles of combination chemotherapy without dose reduction starting from January to May 2022.
Table 1
| Medication | Neoadjuvant chemotherapy dosing | Post-surgical chemotherapy |
|---|---|---|
| vinCRIStine (ONCOVIN) | 2 mg in sodium chloride 0.9% 50 mL | 2 mg in sodium chloride 0.9% 50 mL |
| DOXOrubicin (ADRIAMYCIN) | Chemotherapy injection 167 mg 83.5 mL | Chemotherapy injection 158 mg 79 mL |
| Cyclophosphamide (CYTOXAN) | 2,676 mg & mesna (MESNEX) 535 mg in sodium chloride 0.9% 500 mL | 2,532 mg, mesna (MESNEX) 506 mg in sodium chloride 0.9% 1,000 mL chemotherapy infusion |
| Ifosfamide (IFEX) | 4,010 mg & mesna (MESNEX) 803 mg in sodium chloride 0.9% 500 mL | 3,800 mg, mesna (MESNEX) 760 mg in sodium chloride 0.9% 500 mL chemotherapy infusion |
| Etoposide (VEPESID) | 224 mg in sodium chloride 0.9% 500 mL (starting dose 212) | 212 mg in sodium chloride 0.9% 500 mL chemotherapy infusion |
After the first two complete round of VAC-IE, the patient experienced severe pancytopenia and symptomatic anemia which required admission to the hospital and 1 unit of packed red blood cells. The patient required 6 units of packed red blood cells throughout her chemotherapy treatment as an outpatient prior to surgery. The patient reported relief in her abdominal pain post-chemotherapy. Restaging imaging after 4 months (8 cycles) of chemotherapy demonstrated a decrease in size of the mass with no evidence of distant disease. However, on imaging, there was still significant right ureteral and partial bladder involvement (Figure 2). The patient was again discussed at the multidisciplinary sarcoma tumor board. Given the continued radiologic response seen on cross-sectional imaging as well as her excellent performance status, the decision was made to proceed with attempt at operative resection with surgical oncology along with urology.
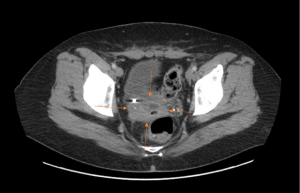
In June 2022, the patient underwent operative exploration. She underwent radical resection of the right pelvic Ewing sarcoma along with right ureterolysis and partial cystectomy and ureteral reimplantation with Biori flap and ureteral stent placement. Frozen sections of the right iliac, right anterior lateral bladder wall, urinary bladder-right ureteral orifice, and anterior vaginal wall were collected and sent to pathology during the procedure for margin assessment, all of which were negative for malignancy. On final pathology, histologically, all sections were found to have significant treatment effect with no residual tumor tissue identified. The patient was deemed as having a complete pathologic response to therapy.
Post-operatively, the patient recovered from surgery without incident. The patient was given 50 mg tramadol for breakthrough pain for 1 week. During a follow-up visit, a slightly reduced ejection fraction was noted on echocardiogram, which was likely a consequence of the systemic regimen. The patient is currently being seen by a cardiologist for follow-up. After discussion at the multidisciplinary sarcoma tumor board, the decision was made to proceed with six additional rounds of dose-reduced chemotherapy, despite the complete pathologic response. The patient’s dose-reduced chemotherapy regimen is listed in Table 1. The patient’s adriamycin was replaced to dactinomycin due to physician preference. As of August 2023, the patient being followed by outpatient oncology and has shown no signs of reoccurrence or long-term toxicity effects from the VAC-IE regimen.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
This report describes a rare case of pelvic EES which demonstrated a complete pathologic response after 8 cycles of VAC-IE chemotherapy.
Strength and limitations
This study gives evidence of VAC-IE being an effective and tolerable regimen for EES in adults when careful monitoring is performed. This case gives an example of a patient who received treatment in an efficient manner. This patient’s medical course was extremely well documented and her associated symptoms and side effects were well documented. However, there is little data on the effects of VAC-IE in adult populations, as Ewing sarcoma is a condition primarily seen in children and young adults (3). However, given the unique nature of this case report, there is limited reported data to which the findings of this case can be appropriately compared.
Comparison with similar research
There have been a small number of studies that have evaluated the tolerability and feasibility of VAC-IE usage in adults (4,5). One study showed that there was no significant difference in toxicity events in individuals older or younger than 30 years old, with minimal need of dose reductions (6). Additionally, there is one case report documenting a 30-year-old female who presented with EES of the mediastinum. This patient was also treated with VAC-IE, experienced complete remission, and was free of reoccurrence for 21 months as of the publishing of the report (7). The patient happened to only experience minimal pancytopenia and anemia, which did not require transfusion (7). A cohort study that looked for prognostic factors and clinical outcomes of EES found that an unresectable tumor should be downstaged with chemotherapy as first line treatment (8). These papers give support to the findings in the report. VAC-IE treatment, when monitored closely, can be an effective and safe treatments in adults.
Explanation of findings
As VAC-IE has been the conventional treatment for ES in children, there has been significant research efforts put into the safety and tolerability of this regimen in that age group (3,9). However, there has been concern in using this regimen in adults due to risk of potential side effect such as severe pancytopenia (6). This report, however, gives support to the fact that some patient may have acceptable toxicity with significant histologic response, provided there is close monitoring of adverse side effects.
Implications and actions needed
While previous reports give evidence to the utility and tolerability or VAC-IE in adults, there still is not enough evidence to support VAC-IE as standard of care treatment for ES in adults without serious concern for tolerability. Randomized controlled trials are difficult to assess this rare incidence disease. However, basket trials may assess this need with a protocolized approach to obtaining higher level data. Such studies are required to identify if VAC-IE should be the standard regiment in adults with ES.
Conclusions
As pelvic EES is rare, there is very little data to guide a standard of care treatment protocol. Therefore, the treatment of osseous and EES are typically similar. For Ewing sarcomas in children, alternating vincristine sulfate, doxorubicin hydrochloride (adriamycin), and cyclophosphamide, followed by ifosfamide and etoposide phosphate is a well-accepted chemotherapy regimen. This report highlights an exceptional response to neoadjuvant chemotherapy for EES. Further work is required to enhance response rates to Ewing sarcoma, specifically in the adult population.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-23-36/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-23-36/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-23-36/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gamberi G, Cocchi S, Benini S, et al. Molecular diagnosis in Ewing family tumors: the Rizzoli experience--222 consecutive cases in four years. J Mol Diagn 2011;13:313-24. [Crossref] [PubMed]
- Natarajan MV, Sameer MM, Bose JC, et al. Surgical management of pelvic Ewing's sarcoma. Indian J Orthop 2010;44:397-401. [Crossref] [PubMed]
- Zöllner SK, Amatruda JF, Bauer S, et al. Ewing Sarcoma-Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J Clin Med 2021;10:1685. [Crossref] [PubMed]
- Lu E, Ryan CW, Bassale S, et al. Feasibility of Treating Adults with Ewing or Ewing-Like Sarcoma with Interval-Compressed Vincristine, Doxorubicin, and Cyclophosphamide Alternating with Ifosfamide and Etoposide. Oncologist 2020;25:150-5. [Crossref] [PubMed]
- Houdek MT, Heidenreich MJ, Ahmed SK, et al. Treatment outcomes of extraskeletal Ewing sarcoma. J Surg Oncol 2023;128:105-10. [Crossref] [PubMed]
- Caltavituro A, Buonaiuto R, Salomone F, et al. Extraskeletal Ewing's sarcoma of the mediastinum: Case report. Front Oncol 2023;13:1074378. [Crossref] [PubMed]
- Palumbo R, Palmeri S, Gatti C, et al. Combination chemotherapy using vincristine, adriamycin, cyclophosphamide (VAC) alternating with ifosfamide and etoposide (IE) for advanced soft tissue sarcomas: a phase II study. Oncol Rep 1998;5:69-72. [Crossref] [PubMed]
- Mathew J, Arjunan R, Dasappa A, et al. Prognostic Factors and Clinical Outcomes in Extraskeletal Ewing Sarcoma: A Cohort Study. Ann Surg Oncol 2023;30:3084-94. [Crossref] [PubMed]
- Corvest V, Marec-Bérard P, Lervat C, et al. Late toxicity comparison of alkylating-based maintenance regimen with cyclophosphamide (VAC) vs ifosfamide (VAI) in Ewing sarcoma survivors treated in the randomized clinical trial Euro-EWING99-R1 in France. Int J Cancer 2023;152:1659-67. [Crossref] [PubMed]
Cite this article as: Mengesha H, Chawla A. Complete pathological response of pelvic Ewing sarcoma in adult female—a case report. AME Med J 2024;9:10.

