
A large spontaneous extrahepatic portosystemic shunt in a cirrhotic patient: from main portal vein to right renal vein
Introduction
Spontaneous portosystemic collateral vessels are frequently observed in liver cirrhosis with portal hypertension. Common types of spontaneous portosystemic collateral vessels in these patients include gastro-oesophageal varices, para-oesophageal veins, gastro-renal or splenorenal shunts, and para-umbilical veins (1). In this case report, we illustrated a very rare type of spontaneous portosystemic shunt originating from the main portal vein to right renal vein.
Case presentation
On January 20, 2014, a 59-year-old male was admitted to our department due to recurrent abdominal distension and weakness for about 5 years and intermittent fever for about one month. He had a 5-year history of alcoholic liver cirrhosis and a 20-year history of diabetes. He drunk white wine with a dose of 50 g per day for more than 30 years. At his admission, the temperature was 38.5 °C, heart rate was 118 b.p.m., and blood pressure was 143/58 mmHg. On physical examinations, there was mild edema of both lower limbs. On laboratory tests, hepatitis B virus surface antigen and hepatitis C virus antibody were negative, alpha fetoprotein (AFP) level was 2.66 IU/mL (0–6.7 IU/mL), white blood cell (WBC) was 5.6×109/L (4×109–10×109/L), percentage of neutrophil was 85.8% (50–70%), hemoglobin concentration was 86 g/L (110–170 g/L), platelet count (PLT) was 162×109/L (100×109–300×109/L), total bilirubin (TBIL) was 17.5 µmol/L (0–20.5 µmol/L), alanine aminotransferase (ALT) was 19 U/L (9–72 U/L), aspartate aminotransferase (AST) was 32 U/L (8–50 U/L), alkaline phosphatase (ALP) was 95 U/L (38–126 U/L), albumin was 25.2 g/L (37–53 g/L), serum creatinine was 59.6 µmol/L (42–133 µmol/L), serum sodium was 125.5 mmol/L (130– 150 mmol/L), prothrombin time (PT) was 15.5 seconds (11.5–14.5 seconds), and international normalized ratio (INR) was 1.23. On axial contrast-enhanced computed tomography scans, liver surface was irregular, spleen was remarkably enlarged, and a large tortuous collateral vessel was communicated between main portal vein and right renal vein (Figure 1). After a written informed consent from this patient and his relatives was obtained, an upper gastrointestinal endoscopy was performed, which demonstrated mild esophageal varices and portal hypertensive gastropathy. After intravenous infusion of ceftriaxone and hepatoprotective drugs were given for 5 days, he was discharged.
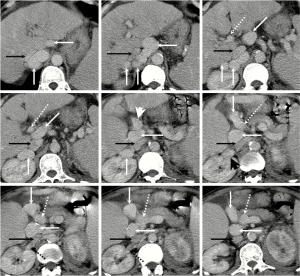
On May 14, 2014, he was re-admitted to our department due to recurrent fever and unconsciousness for 2 days. At this admission, the temperature was 38.8 °C, heart rate was 80 b.p.m., and blood pressure was 179/80 mmHg. On laboratory tests, blood ammonia was 73 µmol/L (9– 54 µmol/L), WBC was 7.5×109/L, percentage of neutrophil was 89.2%, hemoglobin concentration was 90 g/L, PLT was 99×109/L, TBIL was 27.8 µmol/L, ALT was 16 U/L, AST was 22 U/L, ALP was 63 U/L, albumin was 28.5 g/L, serum creatinine was 91 µmol/L, serum sodium was 123.6 mmol/L, PT was 16.3 seconds, INR was 1.31, and AFP was 3.37 IU/mL. MELD score was 11.27 points. Hepatic encephalopathy (HE) and infection were considered. After intravenous infusion of ceftriaxone, L-ornithine-L-aspartate, and hepatoprotective drugs were given for 3 days, his consciousness and temperature became normal with a blood ammonia level of 54 µmol/L. Then, he was discharged.
On October 7, 2014, he was re-admitted to our department due to massive haematemesis and unconsciousness for one day. On laboratory tests, hemoglobin concentration was 51 g/L, WBC was 5.3×109/L, percentage of neutrophil was 78.9%, PLT was 114×109/L, blood ammonia was 34 µmol/L, TBIL was 32.7 µmol/L, ALT was 99 U/L, AST was 178 U/L, ALP was 42 U/L, albumin was 21.7 g/L, serum creatinine was 129.8 µmol/L, serum sodium was 138.2 mmol/L, PT was 15.6 seconds, INR was 1.28, and AFP was 1.88 IU/mL. MELD score was 15.02 points. After a written informed consent from this patient and his relatives was obtained, an upper gastrointestinal endoscopy was performed again, which demonstrated mild esophageal varices and massive duodenal ulcer. After transfusion of red blood cells and pharmacological treatments, including esomeprazole, L-ornithin-L-aspartate, and hepatoprotective drugs, etc., repeated haematemesis and hemafecia remained. Considering the peri-operative risk, his relatives refused surgery. He died on October 12, 2014.
Discussion
The causes for the development of this large collateral vessel were uncertain in our case. Because we did not have any prior CT scans for this patient, we were not sure about when this collateral vessel developed. We had two major considerations, as follows. The first consideration was that this collateral vessel was acquired. If so, embryonic channels were reopened in the setting of portal hypertension. Before the occurrence of liver cirrhosis and portal hypertension, this collateral vessel was not existent. With a gradual increase in the portal pressure, portal blood was spontaneously diverted into renal vein through this collateral vessel.
The second consideration was that this collateral vessel was inherent or congenital. According to the review by Sokollik et al. (2), congenital portosystemic shunt is divided into five types: (I) extrahepatic congenital portosystemic shunt type I with absence or severe hypoplasia of intrahepatic portal venous system; (II) extrahepatic congenital portosystemic shunt type II with maintained intrahepatic portal venous system; (III) intrahepatic congenital portosystemic shunt within the left lobe; (IV) intrahepatic congenital portosystemic shunt within the right lobe; and (V) patent ductus venosus (2). According to the review by Gupta et al. (3), extrahepatic congenital portosystemic shunt type I is further classified as two subtypes: (Ia) splenic vein and superior mesenteric vein drain separately into the inferior vena cava; and (Ib) splenic vein and superior mesenteric vein form a common channel before draining into the inferior vena cava. Extrahepatic congenital portosystemic shunt type II is further classified as three subtypes: (IIa) the portosystemic shunt arises from intrahepatic portal vein branches; (IIb) the portosystemic shunt arises from main portal vein; and (IIc) the portosystemic shunt arises from gastric, mesenteric, or splenic veins. If this case was attributed to the congenital extrahepatic portosystemic shunt, it should be extrahepatic congenital portosystemic shunt type IIb. But it should be noted that the extrahepatic congenital portosystemic shunt should be observed on CT scans obtained at the childhood or at the time when he was not cirrhotic. If the evidence was lacking, congenital portosystemic shunt should be often accompanied with cardiac anomalies.
Our case did not develop any episodes of variceal bleeding or presented with large esophageal varices, but developed HE. This might be associated with the presence of such a large spontaneous portosystemic shunt, which decreased the portal pressure and the liver detoxification. However, the clinical significance of spontaneous portosystemic shunt in cirrhotic patients remained controversial. The first issue was whether or not spontaneous portosystemic shunt decreased the incidence of esophageal varices or variceal bleeding. The second issue was whether or not spontaneous portosystemic shunt increased the incidence of HE. Some researchers suggested no association between spontaneous portosystemic shunt and esophageal varices. In an early study, Rousselot et al. explored the effects of natural portal-systemic shunting in 203 patients, including 135 cirrhotic patients, 15 patients with extrahepatic obstruction of portal system, and 53 patients without any evidence of pathology in the portal system (4). They suggested that the presence of large portosystemic shunts was not associated with the reduction in the portal pressure and incidence or severity of variceal bleeding. Lam et al. compared the incidence of variceal hemorrhage among cases of chronic liver diseases with and without large spontaneous shunts (5). They found a similar proportion of variceal hemorrhage between them. In a retrospective study of 20 patients with large self-established portosystemic shunts, Aseni et al. also concluded that the risk of bleeding was not correlated with presence of massive spontaneous portosystemic shunt (6). By contrast, other researchers suggested that spontaneous portosystemic shunt was a protective factor for the occurrence of esophageal varices and that spontaneous portosystemic shunt could not influence the development of HE. In an early study, Wexler et al. described that a single large spontaneous portosystemic shunt prevented from the development of esophageal varices in six patients with biopsy-proven liver cirrhosis (7). However, one of them presented with severe HE. Iannello et al. also reported that a patient with liver cirrhosis had a large spontaneous splenorenal shunt without any varices at endoscopy or HE (8). Tarantino et al. also confirmed that only one of 15 cirrhotic patients with splenorenal shunts had large esophageal varices (9), but the risk of HE was similar between patients with and without splenorenal shunts. Culafic et al. reported that a case did not have any episodes of HE or variceal bleeding despite the presence of large spontaneous portosystemic shunt (10). Collectively, the heterogeneous findings among studies might be explained by the discrepancy in the sample size and diameter and type of portosystemic shunt. Indeed, Riggio et al. have confirmed the role of large spontaneous portosystemic shunt in the development of persistent HE (11). However, they also acknowledged that two patients with splenic- or mesenteric-renal shunt did not have any episodes of HE and four patients with persistent HE did not have large portosystemic shunts. Thus, other factors should be important in determining the occurrence of HE.
Our case did not receive any interventions for this large collateral, because portosystemic shunt related encephalopathy was readily resolved. Similarly, as recently reported by our team, a case with a large spontaneous intrahepatic portosystemic shunt had no episodes of overt HE and did not receive any therapeutic interventions for portosystemic shunts (12). If there were recurrent of persistent episodes of HE in patients with large portosystemic shunts, angiographic embolization should be considered. In a multicenter European study by Laleman et al., 37 patients with refractory HE were treated with embolization of large spontaneous portosystemic shunts (13). In a single-center study from Mayo Clinic, Rochester, 23 patients with severe persistent HE were also treated with embolization of large spontaneous portosystemic shunts (14). In a single-center case-control study from South Korea, 17 patients with recurrent HE underwent embolization of spontaneous portosystemic shunt (15). Taken together, these studies confirmed the efficacy and safety of embolization of spontaneous portosystemic shunt in patients with recurrent or persistent HE.
In conclusion, this case report demonstrated a very rare case with a large spontaneous extrahepatic portosystemic shunt from main portal vein to right renal vein in a cirrhotic patient.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.02.04). Dr. Qi serves as an Editor-in-Chief of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arora A, Rajesh S, Meenakshi YS, et al. Spectrum of hepatofugal collateral pathways in portal hypertension: an illustrated radiological review. Insights Imaging 2015;6:559-72. [Crossref] [PubMed]
- Sokollik C, Bandsma RH, Gana JC, et al. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr 2013;56:675-81. [Crossref] [PubMed]
- Gupta P, Sinha A, Sodhi KS, et al. Congenital Extrahepatic Portosystemic Shunts: Spectrum of Findings on Ultrasound, Computed Tomography, and Magnetic Resonance Imaging. Radiol Res Pract 2015;2015:181958. [Crossref] [PubMed]
- Rousselot LM, Moreno AH, Panke WF. Studies on portal hypertension. IV. The clinical and physiopathologic significance of self-established (nonsurgical) portal systemic venous shunts. Ann Surg 1959;150:384-412. [Crossref] [PubMed]
- Lam KC, Juttner HU, Reynolds TB. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci 1981;26:346-52. [Crossref] [PubMed]
- Aseni P, Beati C, Brambilla G, et al. Does large spontaneous portal systemic shunt in cirrhosis protect from the risk of gastroesophageal bleeding? J Clin Gastroenterol 1986;8:235-8. [Crossref] [PubMed]
- Wexler MJ, MacLean LD. Massive spontaneous portal-systemic shunting without varices. Arch Surg 1975;110:995-1003. [Crossref] [PubMed]
- Iannello S, Libertini L, Martini R, et al. A large spontaneous splenorenal shunt in a patient with liver cirrhosis and uncomplicated portal hypertension. Dig Dis 1999;17:248-55. [Crossref] [PubMed]
- Tarantino G, Citro V, Conca P, et al. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol 2009;9:89. [Crossref] [PubMed]
- Culafic D, Perisic M, Vojinovic-Culafic V, et al. Spontaneous splenorenal shunt in a patient with liver cirrhosis and hypertrophic caudal lobe. J Gastrointestin Liver Dis 2006;15:289-92. [PubMed]
- Riggio O, Efrati C, Catalano C, et al. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case-control study. Hepatology 2005;42:1158-65. [Crossref] [PubMed]
- Qi X, Ye C, Hou Y, et al. A large spontaneous intrahepatic portosystemic shunt in a cirrhotic patient. Intractable Rare Dis Res 2016;5:58-60. [Crossref] [PubMed]
- Laleman W, Simon-Talero M, Maleux G, et al. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology 2013;57:2448-57. [Crossref] [PubMed]
- Singh S, Kamath PS, Andrews JC, et al. Embolization of spontaneous portosystemic shunts for management of severe persistent hepatic encephalopathy. Hepatology 2014;59:735-6. [Crossref] [PubMed]
- An J, Kim KW, Han S, et al. Improvement in survival associated with embolisation of spontaneous portosystemic shunt in patients with recurrent hepatic encephalopathy. Aliment Pharmacol Ther 2014;39:1418-26. [Crossref] [PubMed]
Cite this article as: Bao Y, Qi X, Shao X, Guo X. A large spontaneous extrahepatic portosystemic shunt in a cirrhotic patient: from main portal vein to right renal vein. AME Med J 2017;2:17.

