
An extremely increased CA19-9 level due to common bile duct stone: a case report
Introduction
Serum carbohydrate antigen 19-9 (CA19-9) is mainly used for the diagnosis and prognostic assessment of pancreatobiliary neoplasms. A CA19-9 level of >100 U/mL is more likely to indicate the presence of malignant diseases, especially early stage of pancreatic cancer (1). The specificity of CA19-9 level >1,000 U/mL for pancreatic cancer was 99% (2). However, a high CA19-9 level of >1,000 U/mL was often found in some benign diseases, such as common bile duct stones, acute cholangitis, acute pancreatitis, diabetes, and liver cirrhosis (3). Herein, we reported a case with an extremely increased CA19-9 level due to benign common bile duct stones and gallstones.
Case presentation
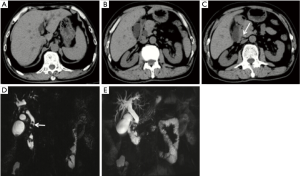
On September 12, 2016, a 63-year-old male was admitted to our hospital due to intermittent right upper quadrant abdominal pain for 1 year. No radiating pain, chills, fever, jaundice, nausea, or vomiting was observed. He did not have any other medical history. On the physical examinations, there was right upper quadrant tenderness without any rebound. The laboratory data were as follows: white blood cell (WBC) was 4.3×109/L (normal range: (3.5×109– 9.5×109/L); percentage of neutrophils was 54.9% (normal range: 40–75%); alanine transaminase (ALT) was 52.31 U/L (normal range: 9–50 U/L); aspartate transaminase (AST) was 47.23 U/L (normal range: 15–40 U/L); total bilirubin (TBIL) was 33.4 µmol/L (normal range: 5.1–22.2 µmol/L); direct bilirubin (DBIL) was 18.5 µmol/L (normal range: 0–8.6 µmol/L); gamma-glutamyl transpeptidase (GGT) was 455.22 U/L (normal range: 10–60 U/L); alkaline phosphatase (ALP) was 408.22 U/L (normal range: 45– 125 U/L); CA19-9 was more than 1,000.0 U/mL (normal range: 0–37 U/mL); CA-50 was 466.34 IU/mL (normal range: 0–25 IU/mL); CA24-2 was more than 200 IU/mL (normal range: 0–20 IU/mL). Abdominal computer tomography scan demonstrated intrahepatic bile duct and common bile duct dilatation, gallbladder enlargement, nodular high density shadow at the bottom of gallbladder and common bile duct, and no abnormality of pancreatic morphology and density (Figure 1A,B,C). Magnetic resonance cholangiopancreatography (MRCP) demonstrated left and right intrahepatic bile duct dilatation, “deadwood”-like changes in the distal intrahepatic bile duct, multiple circular filling defect at the lower end of the common bile duct, no obvious pancreatic duct dilatation, gallbladder enlargement, multiple small circular filling defect at the bottom of the gallbladder (Figure 1D,E). Thus, common bile duct stones, lower bile duct obstruction, and gallbladder stones were diagnosed.
On September 18, 2016, endoscopic retrograde cholangiopancreatography (ERCP), endoscopic sphincterotomy, endoscopic papillary balloon dilatation, the remove of calculus from common bile duct, and endoscopic nasobiliary drainage were successfully performed. Finally, two black-brown stones were removed.
On September 19, 2016, the laboratory data were as follows: WBC was 9.2×109/L (normal range: 3.5×109–9.5×109/L); percentage of neutrophils was 82.1% (normal range: 40–75%); TBIL was 30.2 µmol/L (normal range: 5.1–22.2 µmol/L); DBIL was 15 µmol/L (normal range: 0–8.6 µmol/L); ALT and AST back to the normal range. GGT and ALP fell to 325.51 U/L (normal range: 10– 60 U/L) and 277.79 U/L (normal range: 45–125 U/L), respectively; CA19-9 and CA-50 sharply fell to 124.7 U/mL (normal range: 0–37 U/mL) and 43.83 IU/mL (normal range: 0–25 IU/mL), respectively.
On September 21, 2016, the laboratory data were as follows: TBIL was 24.7 µmol/L (normal range: 5.1– 22.2 µmol/L); DBIL was 13.1 µmol/L (normal range: 0– 8.6 µmol/L); GGT was 288.51 U/L (normal range: 10– 60 U/L); ALP was 251.23 U/L (normal range: 45–125 U/L); CA199 was 78.62 U/mL (normal range: 0–37 U/mL); CA-50 was 25.76 IU/mL (normal range: 0–25 IU/mL). WBC, percentage of neutrophils, ALT, AST, and CA24-2 level were within the normal range.
On September 22, 2016, the patient was discharged without any remarkable complaints. On December 15, 2016, a telephone follow-up was performed. Now, he is very well, but refuse the CA19-9 test again.
Discussion
CA19-9 is mainly distributed in the fetal pancreas, gallbladder, liver, intestine and other tissues, and in healthy adult pancreas, bile duct epithelium, etc. In healthy people, CA19-9 is secreted and synthesized by bile duct and pancreatic duct epithelial cells, transported to the common bile duct and pancreatic duct, and then discharged through the duodenum. Under normal circumstances, CA19-9 in the bile duct cells rarely released to the peripheral blood. It is up-regulated in some malignant tumors, including pancreatic cancer, gastric cancer, gallbladder carcinoma, hepatocellular carcinoma, colorectal carcinoma, and ovarian cancer as well as benign diseases such as pancreatitis, cholangitis, and choledocholithiasis (4-6). Occasionally, bile duct stones, inflammation and other benign diseases can lead to markedly elevated levels of serum CA19-9 (7-13).
Recently, a case about acute cholecystitis with a significantly elevated levels of serum CA19-9 (19,392 U/mL) was diagnosed based on the PET-CT findings. After anti-inflammatory and cholecystectomy, CA19-9 returned to the normal range. Acute cholecystitis with a significantly elevated levels of serum CA19-9 (>10,000 U/mL) is rare (14). The mechanisms of CA19-9 elevation in benign biliary disease are as follows (4):
- Excessive bile duct pressure stimulated bile duct cells to produce more CA19-9;
- Inflammation stimulated epithelial hyperplasia, leading to an increase in cells secreting CA19-9;
- Biliary obstruction and cholestasis decreased the clearance of CA19-9;
- When the bile duct stones and inflammation subsided, the bile duct cells normalized, and CA19-9 also returned to normal levels.
In our case, a high level of CA19-9 was mainly due to the obstruction of common bile duct stones. Elevated serum TBIL, ALP, and GGT also suggested the presence of biliary stones and obstruction. After the obstruction and stones were removed, CA19-9 level decreased significantly. The elevated CA19-9 was not caused by tumor tissue secretion. If an elevated CA19-9 level was caused by cancer cells, it would not be significantly reduced after relieving obstruction.
An elevated CA19-9 level has been also observed in benign non-biliary diseases. Howaizi et al. (15) reported a case of markedly raised level of CA19-9 associated with heavy tea consumption. The patient presented with epigastric pain, anorexia, nausea, and over-consumption of warm black tea for several months (1.5–2 liters/day). CA19-9 level was 1,432 UI/mL. Other examinations were normal. The patient was advised to stop tea consumption. Four weeks later, she became asymptomatic and CA19-9 level dropped to 42 UI/mL. A re-challenge test was then attempted. The patient restarted tea consumption as previously. Four weeks later, CA19-9 level increased to 745 UI/mL followed by a fall to 25 UI/mL one month after withdrawal. Uyanik et al. (16) reported two cases with abnormal levels of CA19-9 from the same family. CA19-9 levels were slightly elevated in the mother (89.90 U/mL) and her daughter (123.92 U/mL). There was no clinical pathology. Therefore, healthy people can exceed normal range of upper limit. Han et al. (17) reported that a case of pulmonary sequestration leads to benign disease in the left upper abdominal distension and CA19-9 elevation (790.6 U/mL). Han et al. (18) reported that chronic bronchitis with fungal infection presenting with a marked elevation of CA19-9 from 494.39 to 1,212.04 U/mL. Pyeon et al. (19) presented with an abnormally high level of CA19-9 (2,753 U/mL, normal range: 0–27 U/mL) associated with benign mucinous cystadenoma. Filipovic et al. (20) reported a case of elevated of CA19-9 level (3,500 U/mL) associated with ureteric calculi induced hydronephrosis. Sapmaz et al. (21) reported a case of approximately 30-fold increased CA19-9 in autoimmune hepatitis. The patients had no evidence of any malignant disease in pancreatobiliary or gastrointestinal tracts. CA19-9 levels decreased to normal levels with immunosuppressive treatment. In addition, abnormally elevated CA19-9 was also found in interstitial lung disease, collagen vascular disease, congestive heart disease, endometriosis, colonic diverticulitis, diabetes mellitus, hypothyroidism, and rheumatoid arthritis, etc. (14-16).
Therefore, CA19-9 might not be used as an effective diagnostic criterion. In many pancreatic cancer patients, CA19-9 were within the normal range (CA19-9 <37 U/mL). In patients with suspicious pancreatic head masses and CA19-9 levels <37 U/mL, age distribution, abdominal pain and bilirubin might be useful in the differentiation between malignant and benign diseases. Pancreatic head malignancies were more likely to cause obstructive jaundice (22).
In conclusion, the change in the serum CA19-9 levels over time and imaging studies were important to distinguish between benign and malignant lesions. If CA19-9 declines rapidly, inflammation and calculus may be considered.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.02.02). Dr. Qi serves as an Editor-in-Chief of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saluja SS, Sharma R, Pal S, et al. Differentiation between benign and malignant hilar obstructions using laboratory and radiological investigations: a prospective study. HPB (Oxford) 2007;9:373-82. [Crossref] [PubMed]
- Steinberg W. The clinical utility of the CA 19-9 tumor-associated antigen. Am J Gastroenterol 1990;85:350-5. [PubMed]
- Mimidis K, Anagnostoulis S, Iakovidis C, et al. Remarkably elevated serum levels of carbohydrate antigen 19-9 in cystic duct and common bile duct lithiasis. J Gastrointestin Liver Dis 2008;17:111-2. [PubMed]
- Sheen-Chen SM, Sun CK, Liu YW, et al. Extremely elevated CA19-9 in acute cholangitis. Dig Dis Sci 2007;52:3140-2. [Crossref] [PubMed]
- Ong SL, Sachdeva A, Garcea G, et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci 2008;53:3213-7. [Crossref] [PubMed]
- Morris-Stiff G, Teli M, Jardine N, et al. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int 2009;8:620-6. [PubMed]
- Robertson AG, Davidson BR. Mirizzi syndrome complicating an anomalous biliary tract: a novel cause of a hugely elevated CA19-9. Eur J Gastroenterol Hepatol 2007;19:167-9. [Crossref] [PubMed]
- Murray MD, Burton FR, Di Bisceglie AM. Markedly elevated serum CA 19-9 levels in association with a benign biliary stricture due to primary sclerosing cholangitis. J Clin Gastroenterol 2007;41:115-7. [Crossref] [PubMed]
- Katsanos KH, Kitsanou M, Christodoulou DK, et al. High CA 19-9 levels in benign biliary tract diseases. Report of four cases and review of the literature. Eur J Intern Med 2002;13:132-5. [Crossref] [PubMed]
- Albert MB, Steinberg WM, Henry JP. Elevated serum levels of tumor marker CA19-9 in acute cholangitis. Dig Dis Sci 1988;33:1223-5. [Crossref] [PubMed]
- Lin CL, Changchien CS, Chen YS. Mirizzi's syndrome with a high CA19-9 level mimicking cholangiocarcinoma. Am J Gastroenterol 1997;92:2309-10. [PubMed]
- Ogawa T, Yokoi H, Kawarada Y. A case of inflammatory pseudotumor of the liver causing elevated serum CA19-9 levels. Am J Gastroenterol 1998;93:2551-5. [Crossref] [PubMed]
- Trompetas V, Panagopoulos E, Ramantanis G. Gall-bladder agenesis presenting with obstructive jaundice and elevated CA 19-9. Acta Chir Belg 2004;104:347-9. [Crossref] [PubMed]
- Akimoto S, Banshodani M, Nishihara M, et al. Acute Cholecystitis with Significantly Elevated Levels of Serum Carbohydrate Antigen 19-9. Case Rep Gastroenterol 2016;10:410-6. [Crossref] [PubMed]
- Howaizi M, Abboura M, Krespine C, et al. A new cause for CA19.9 elevation: heavy tea consumption. Gut 2003;52:913-4. [Crossref] [PubMed]
- Uyanik M, Sertoglu E, Serdar MA, et al. Two cases of the same family with the unusual elevation of CA19-9 levels. Acta Med Port 2014;27:657-60. [Crossref] [PubMed]
- Han P, Luo Y, Tian D, et al. Pulmonary sequestration presenting with left upper abdominal bloating and marked elevation of serum carbohydrate antigen 19-9: A case report. Oncol Lett 2014;7:1493-6. [PubMed]
- Han P, Yan W, Luo Y, et al. Chronic bronchitis with fungal infection presenting with marked elevation of serum carbohydrate antigen 19-9: a case report. Int J Clin Exp Pathol 2014;7:6307-12. [PubMed]
- Pyeon SY, Park JY, Ki KD, et al. Abnormally high level of CA-19-9 in a benign ovarian cyst. Obstet Gynecol Sci 2015;58:530-2. [Crossref] [PubMed]
- Filipovic B, Milinić N, Gacic J, et al. Benign Hydronephrosis and Elevated of Serum Levels of Carbohydrate Antigen CA 19-9: A Case Report. Am J Case Rep 2016;17:395-7. [Crossref] [PubMed]
- Sapmaz F, Kalkan IH, Kısa Ü, et al. A very rare cause of markedly elevated CA 19.9: Autoimmune hepatitis. Acta Clin Belg 2016;1-3. [Epub ahead of print]. [PubMed]
- Jin X, Wu Y. Diagnostic utility of clinical and biochemical parameters in pancreatic head malignancy patients with normal carbohydrate antigen 19-9 levels. Afr Health Sci 2015;15:123-30. [Crossref] [PubMed]
Cite this article as: Wang X, Qi X, Li H, Shao X, Guo X. An extremely increased CA19-9 level due to common bile duct stone: a case report. AME Med J 2017;2:18.

