Pylorus-preserving gastrectomy versus distal gastrectomy in oncology for the patient with early gastric cancer: a meta-analysis
Introduction
Due to the advances in diagnostic modalities, the gastric cancer is allowed to be detected earlier and the occurrence of early gastric cancer (EGC) has been raising, even in asymptomatic patients. Patients who have EGC usually get a satisfactory prognosis after surgical treatment, with overall 3-year survival rate of 97.8% and disease-specific 3-year survival rate of 99.3% reported by Jiang et al. (1) This has drawn attention of surgeons to function-preserving surgery which can minimize postgastrectomy problems such as dumping syndrome, alkaline reflux, gastroesophagitis, nutritional deficit, and weight loss, while maintaining an excellent level of radicality (2).
Pylorus-preserving gastrectomy (PPG) which was firstly adopted at the therapy of peptic ulcers with content outcomes by Maki et al. (3) and has since been introduced as a function-preserving treatment for EGC (4-7). So far, many studies have reported that PPG has advantages versus distal gastrectomy (DG) with Billroth I reconstruction by functional conserving (8-10).
Among these studies, the surgical and oncological safety of PPG has still no consensus. Besides, most studies on PPG have only reported fragmentary results, and the patients with long-term results after PPG are not enough to provide credible evaluation for oncological outcome. Thus, we performed a meta-analysis to systematically and objectively assess the possible advantage of PPG comparing with DG in the outcomes of EGC.
Methods
Search strategy
By searching the major medical databases, PubMed, EMBASE, and the Cochrane Library, we identified relevant publications up to March 26, 2016. We used the MeSH form strategy for PubMed as follows: ((stomach neoplasms (Mesh)) OR (stomach neoplasms) OR (stomach neoplasm) OR (stomach carcinoma) OR (stomach cancer) OR (stomach cancers) OR (stomach tumor) OR (cancer of stomach) OR (cancer of the stomach) OR (gastric neoplasms) OR (gastric neoplasm) OR (gastric carcinoma) OR (gastric cancer) OR (gastric cancers) OR (gastric tumor)) AND ((pylorus-preserving gastrectomy) OR (function-preserving gastrectomy)). The publication language was limited in English, Chinese and Japanese.
The references of relevant articles and previous meta-analyses were manually searched to identify additional relevant articles. In order to check for additional studies, we also used authors’ names as search terms and the “related articles” function in PubMed database.
Outcome measures
The primary outcomes were recurrence, metastasis and survival, including overall survival (OS) and recurrence-free survival (RFS). The secondary outcomes were operative time, intraoperative blood loss, postoperative hospital stay, overall complication rate, and number of retrieved lymph nodes. OS was defined from surgery to death for any reason. RFS was defined from surgery to the first occurrence of disease progression or relapse due to the primary cancer.
Inclusion and exclusion criteria
All studies involved in this meta-analysis should meet the following criteria: (I) studies that compared PPG versus conventional DG for EGC; (II) patients diagnosed with primary gastric adenocarcinoma; (III) data that included the primary and secondary outcomes. Two exclusion criteria were applied: (I) gastrointestinal stromal tumors or benign gastric diseases or advanced gastric cancer; (II) publication with overlapped data or insufficient data for the analyses.
Three of the authors (Tao Chen, Ziyu Chen and Li Zhen) assessed the eligibility of all studies searched from the databases according to the selection criteria, independently. The risk of bias in each study was evaluated according to the Newcastle-Ottawa Scale for observational studies.
Data extraction
Three researchers (Tao Chen, Ziyu Chen and Li Zhen) used a structured sheet to extract data from each study, which were then entered into a database. Another author (Xiaolong Qi) reviewed this process. Disagreements were resolved through discussion and consensus of the study team.
Statistical analysis
We used the software Review Manager (RevMan) ver. 5.0 by the Cochrane Collaboration (Nordic Cochrane Center, Copenhagen, Denmark) to conduct this meta-analysis. Odds ratio (OR) for dichotomous variables and weighted mean differences (WMD) for continuous outcome measures were calculated with 95% confidence intervals (CIs). Hazard ratio (HR) was calculated as a summary statistic for censored outcomes (OS and RFS) as described by Tierney et al. (11) Three investigators used Engauge Digitizer version 4.1 to read the Kaplane-Meier curves independently in order to reduce reading variability and estimated the HRs and the 95% CIs. Heterogeneity of the results across studies was evaluated by Higgins I2 and chi-square tests, while an I2 value of greater than 50% and a P value of chi-square less than 0.10 are considered as indicative of substantial heterogeneity. If there is no heterogeneity, fixed-effects model was applied, otherwise, the random effects model was used. We obtained the standard deviation by extracting the estimated standard deviation from the P value, or contacting the authors by e-mail. Begg’s funnel plot was carried out to estimate the potential publication bias in the studies. The risk of bias for cohort studies was assessed by the Newcastle-Ottawa scale, while risk for randomized controlled trials (RCT) was assessed based on the Cochrane risk of bias tool.
Results
In total, 16 articles (8,10,12-25) were included on the basis of our inclusion and exclusion criteria (Figure 1). Two studies (12,17) are multicenter randomized control trial (RCT), others are non-randomized, non-blinded, retrospective cohort studies. Table 1 shows the characteristics of the sixteen included articles are shown in.
Table 1
| Study | Journal | Country | Language | Number of patient (PPG) | Number of patient (DG) | No. 1/5/6/7 lymph node was dissected in PPG |
|---|---|---|---|---|---|---|
| Kodama et al. 1995 | World Journal of Surgery | Japan | English | 35 | 29 | NS/N/Y/Y |
| Sawai et al. 1995 | Am J Surg | Japan | English | 25 | 33 | Y/N/Y/Y |
| Imada et al. 1998 | Surgery | Japan | English | 20 | 25 | N/N/Y/N |
| Zhang et al. 1998 | Arch Surg | Japan | English | 15 | 28 | Y/Y/Y/P |
| Hotta et al. 2001 | Surg Today | Japan | English | 19 | 45 | Y/N/Y/N |
| Shibata et al. 2004 | World J Surg | Japan | English | 36 | 38 | P/Y/Y/P |
| Urushihara et al. 2004 | Surg Endosc | Japan | English | 26 | 26 | NS/NS/NS/NS |
| Nomura et al. 2005 | Japanese Journal of Gastroenterological Surgery | Japan | Japanese | 71 | 262 | Y/P/Y/Y |
| Park et al. 2008 | World Journal of Surgery | Korea | English | 22 | 17 | Y/Y/Y/Y |
| Hu et al. 2010 | Zhonghua Wei Chang Wai Ke Za Zhi | China | Chinese, only survival | 52 | 159 | Y/N/Y/P |
| Ikeguchi et al. 2010 [1] | Surg Today | Japan | English | 46 | 87 | Y/N/Y/Y |
| Ikeguchi et al.2010 [2] | Indian J Surg | Japan | English | 24 | 30 | Y/N/Y/Y |
| Lee et al. 2010 | J Am Coll Surg | Japan | English | 148 | 305 | P/NS/P/P |
| Tomikawa et al. 2012 | Surg Today | Japan | English | 9 | 12 | Y/NS/Y/Y |
| Kim et al. 2013 | Ann Surg Oncol | Korea | English | 21 | 109 | Y/Y/Y/Y |
| Suh et al. 2014 | Annals of Surgery | Korea | English | 116 | 176 | Y/N/Y/Y |
PPG, pylorus-preserving gastrectomy; DG, distal gastrectomy; “N’’, no; “Y”, yes; “P”, partial; “NS”, not stated.
A total of 2,066 patients diagnosed as EGC were analyzed, including 685 patients who underwent PPG, 1,381 patients who underwent DG. The risk of bias of included studies is shown in Tables 2 and 3.
Table 2
| Study (Cohort studies) | Selection (0–4) | Comparability (0–2); outcome (0–3) | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REC | SNEC | AE | DO | SC | AF | AO | FU | AFU | |||
| Sawai et al. 1995 | * | * | * | * | * | * | 6 | ||||
| Imada et al. 1998 | * | * | * | * | * | * | 6 | ||||
| Zhang et al. 1998 | * | * | * | * | * | * | 6 | ||||
| Hotta et al. 2001 | * | * | * | * | * | * | * | 7 | |||
| Urushihara et al. 2004 | * | * | * | * | * | * | 6 | ||||
| Nomura et al. 2005 | * | * | * | * | * | * | 6 | ||||
| Park et al. 2008 | * | * | * | * | * | * | * | 7 | |||
| Hu et al. 2010 | * | * | * | * | * | * | 6 | ||||
| Ikeguchi et al. 2010 [1] | * | * | * | * | * | * | * | 7 | |||
| Ikeguchi et al. 2010 [2] | * | * | * | * | * | * | 6 | ||||
| Lee et al. 2010 | * | * | * | * | * | * | 6 | ||||
| Tomikawa et al. 2012 | * | * | * | * | * | * | 6 | ||||
| Kim et al. 2013 | * | * | * | * | * | * | 6 | ||||
| Suh et al. 2014 | * | * | * | * | * | * | * | 7 | |||
REC, representativeness of the exposed cohort; SNEC, selection of the non-exposed cohort; AE, ascertainment of exposure; DO, demonstration that outcome of interest was not present at start of study; SC, study controls for age, sex, marital status; AF, study controls for any additional factors; AO, assessment of outcome; FU, follow-up long enough for outcomes to occur; AFU, adequacy of follow-up of cohorts. *Asterisk means that the study is satisfied the item, and no asterisk means the opposite situation.
Early outcomes after the surgery
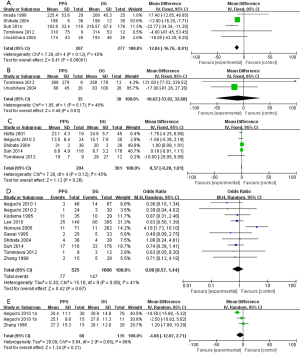
These outcomes include operative time, intraoperative blood loss, postoperative hospital stay, overall complication rate and number of retrieved lymph nodes. The pooled results were shown in Table 4. Shibata et al. (17) reported that the operative time was shorter in PPG than DG (WMD =−12.00; 95% CI: −16.29 to −7.71, P<0.01). Likewise, our analysis of the operative time demonstrated a significant difference between PPG and DG (WMD =−12.84; 95% CI: −16.76 to −8.91, P<0.01) in a fixed effect model (P=0.12, I2 =45%; Figure 2), and PPG took shorter operative time. The intraoperative blood loss was less in PPG than DG, but without statistical significance (WMD =−10.62; 95% CI: −53.92 to 32.68, P=0.63) in a fixed effect model (P=0.17, I2=46%; Figure 2). Additionally, there was no significant difference in postoperative hospital stay (WMD =0.37; 95% CI: −0.28 to 1.01, P=0.26) and overall complication rate (OR =0.90; 95% CI: 0.57 to 1.44, P=0.67) between the two groups in a fixed effect model (P=0.12, I2=45%; Figure 2) and a random effect model (P=0.09, I2=41%; Figure 2). The number of retrieved lymph nodes was lower in PPG compared with DG, though without a statistically significant difference (WMD =−4.68, 95% CI: −12.07 to 2.71, P=0.21) in a random effect model (P =0.05, I2=66%; Figure 2).
Table 4
| Observed outcomes | Number of study | PPG patients | DG patients | HR/OR/WMD (95% CI) | P | Study heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P value | ||||||
| Early outcomes | |||||||
| Operative time | 5 | 207 | 277 | −12.84 (−16.76 to 8.91) | <0.01 | 45 | 0.12 |
| Intraoperative blood loss | 2 | 35 | 38 | −10.62 (−53.92 to 32.68) | 0.63 | 46 | 0.17 |
| Postoperative hospital stay | 5 | 204 | 301 | 0.37 (−0.28 to 1.01) | 0.26 | 45 | 0.12 |
| Overall complication rate | 10 | 525 | 1000 | 0.90 (0.57 to 1.44) | 0.67 | 41 | 0.09 |
| Number of retrieved lymph nodes | 2 | 60 | 115 | −4.68 (−12.07 to 2.71) | 0.21 | 66 | 0.05 |
| Late outcomes | |||||||
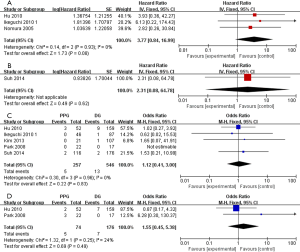
| Overall survival | 3 | 168 | 500 | 3.77 (0.84 to 16.99) | 0.08 | 0 | 0.93 |
| Recurrence-free survival | 1 | 116 | 176 | 2.31 (0.08 to 64.78) | 0.62 | – | – |
| Recurrence | 5 | 257 | 546 | 1.12 (0.41 to 3.00) | 0.83 | 0 | 0.96 |
| Metastasis | 2 | 74 | 176 | 1.55 (0.45 to 5.38) | 0.49 | 24 | 0.25 |
Late outcomes after the surgery
Three studies (19-21) reported OS, only one study (25) investigated RFS. There was not a significant difference in OS with PPG compared with DG (HR =3.77; 95% CI: 0.84 to 16.99, P=0.08) in a fixed effect model (P=0.93, I2=0%; Figure 3). The same non-significant outcome RFS (HR =2.31; 95% CI: 0.08 to 64.78, P=0.62; Figure 3) was noted when including only one data. Additionally, the two comparative surgical approaches did not differ significantly in regards to local recurrence and metastasis (OR =1.12; 95% CI: 0.41 to 3.00, P=0.83; Figure 3); (OR =1.55; 95% CI: 0.45 to 5.38, P=0.49; Figure 3); respectively.
Discussion
Extent of lymphadenectomy
Although many investigators has recommended PPG as a function-preserving surgery for EGC (26), the inconsistency of procedures still exist in different institutions. The main controversy is relation with the extent of lymphadenectomy. Firstly, to prevent from the schemic of pylorus and preserve the vagal nerves, one of the pitfall of PPG is considered to be insufficient lymphadenectomy for N0.5 station, which has potential risks against oncological safety and prompted many to advocated strict and limited indications for PPG (26). However, in Nakajima’s previous study (27), only a 0.2% rate of metastases of No. 5 station in 3,646 case of T1 (mucosal or submucosal) cancer located in middle of the body of stomach. Other retrospective analysis reported that 0.0–0.5% of T1 gastric cancers showed lymph node metastases to No. 5 station (7,28). Based on these dates, Hiki (26) imply that patients who are diagnosed clinically as T1N0 could be candidates for PPG without suprapyloric lymphadenectomy, if the preoperative diagnosis of T1N0 is accurate. In our meta-analysis, eight studies report lymphadenectomy without No. 5 in PPG procedure, conversely only four studies with No. 5 all of which report the preservation of pyloric branch simultaneously except one not. In this exceptional study, Zhang et al. (15) demonstrated No. 5 lymphadenectomy excluding preservation of pyloric branch accompanying PPG didn’t negate these benefits in postoperative gastric function and can be safely performed if infrapyloric artery is preserved. But more studies are need for further verification. Secondly, the dissection of No. 6 lymph node is frequently incomplete in order to reserve the infrapyloric vessels that may also be worrisome in terms of oncologic safety. But in consider of the present and previous studies, No. 6 lymph nodes are sufficiently dissected in PPG procedure compared to DG, and the micrometastasis and macrometastasis are very rare in No. 6 station (8,28). Thirdly, there are still some debates regarding the dissection of No. 7. On the ground of celiac branch anatomic relation with left gastric artery, dissection of No. 7 with the preservation of celiac branch is still a difficult technology which is a battery for the previant of PPG, because in 81.8% of cases, celiac branch lay closer to the left gastric artery (29). In our meta-analysis, only six studies show the preservation of celiac branch. However, for experienced surgeon, even in laparoscopic pylorus-preserving gastrectomy (LAPPG) procedure, the celiac branch can be preserved regardless of its anatomical variation during the dissection of No. 7 lymph nodes (5,29). In brief, more comparative clinical studies are needed to demonstrate the oncological safety of the preservation of the nerves and vessels with or without the extent of No. 5, 6, 7 lymphadenectomy.
Perioperative course
Our meta-analysis demonstrated that PPG take shorter time compared with DG. Other perioperative results, including intraoperative blood loss, postoperative hospital stay, overall complication rate, and number of retrieved lymph nodes, were found to have no significant statistical difference. These results meant PPG had the advantage over DG during perioperative period.
Oncological safety of PPG
The 5-year survival rate for patients with EGC after gastrectomy with radical lymph node dissection ranges from 93% to 98% (30). Morita et al. (9) reported the first overall 5-year survival rate for 611 T1 gastric cancer patients after PPG (96.3%) which seems comparable to the outcome of open DG for EGC. However, the prognosis of PPG comparing with DG is still a contentious issue. Recently, Hiki et al. (31) reported a 98% overall 5-year survival and 0% gastric cancer-related deaths in 305 patients treated using PPG and it demonstrated that PPG may provide a long-term survival benefit for patients with clinically diagnosed T1N0 gastric cancer in the middle one-third of the stomach, only when the accuracy of preoperative diagnosis can be assured.
The results in our meta-analysis revealed that there was no significant difference in OS and RFS between PPG and DG. As for recurrence and metastasis, there was no significant difference between the two operations, either. Previous meta-analysis conducted by Song et al. (32) suggested that PPG provided a better life quality over DG. Thus, PPG might could be considered as a better operation for EGC.
Conclusions
To sum up, comparing with DG, PPG has shorter operative time for EGC. However, other outcomes such as Intraoperative blood loss, postoperative hospital stay, overall complication rate, number of retrieved lymph nodes, OS, RFS, recurrence and metastasis don’t differ significantly. Moreover, higher quality trials are needed to evaluate PPG’s clinical and oncological outcomes.
Acknowledgments
Funding: This work was supported by the Major Program of Science and Technology Program of Guangzhou, No. 201300000087; the Research Fund of Public Welfare in Health Industry, Heath Ministry of China, No. 201402015; the Science and Technology Planning Project of Guangdong Province, No. 2016ZC0072; and the Key Clinical Specialty Discipline Construction Program.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jiang X, Hiki N, Nunobe S, et al. Long-term outcome and survival with laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer. Surg Endosc 2011;25:1182-6. [Crossref] [PubMed]
- Shimizu S, Tada M, Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy 1995;27:27-31. [Crossref] [PubMed]
- Maki T, Shiratori T, Hatafuku T, et al. Pylorus-preserving gastrectomy as an improved operation for gastric ulcer. Surgery 1967;61:838-45. [PubMed]
- Hiki N, Kaminishi M. Pylorus-preserving gastrectomy in gastric cancer surgery--open and laparoscopic approaches. Langenbecks Arch Surg 2005;390:442-7. [Crossref] [PubMed]
- Hiki N, Shimoyama S, Yamaguchi H, et al. Laparoscopy-assisted pylorus-preserving gastrectomy with quality controlled lymph node dissection in gastric cancer operation. J Am Coll Surg 2006;203:162-9. [Crossref] [PubMed]
- Nishikawa K, Kawahara H, Yumiba T, et al. Functional characteristics of the pylorus in patients undergoing pylorus--preserving gastrectomy for early gastric cancer. Surgery 2002;131:613-24. [Crossref] [PubMed]
- Nunobe S, Hiki N, Fukunaga T, et al. Laparoscopy-assisted pylorus-preserving gastrectomy: preservation of vagus nerve and infrapyloric blood flow induces less stasis. World J Surg 2007;31:2335-40. [Crossref] [PubMed]
- Kim BH, Hong SW, Kim JW, et al. Oncologic safety of pylorus-preserving gastrectomy in the aspect of micrometastasis in lymph nodes at stations 5 and 6. Ann Surg Oncol 2014;21:533-8. [Crossref] [PubMed]
- Morita S, Katai H, Saka M, et al. Outcome of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 2008;95:1131-5. [Crossref] [PubMed]
- Park DJ. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg 2008;32:1029-36. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Kodama M, Koyama K, Chida T, et al. Early postoperative evaluation of pylorus-preserving gastrectomy for gastric cancer. World J Surg 1995;19:456-60; discussion 61. [Crossref] [PubMed]
- Sawai K, Takahashi T, Fujioka T, et al. Pylorus-preserving gastrectomy with radical lymph node dissection based on anatomical variations of the infrapyloric artery. Am J Surg 1995;170:285-8. [Crossref] [PubMed]
- Imada T, Rino Y, Takahashi M, et al. Postoperative functional evaluation of pylorus-preserving gastrectomy for early gastric cancer compared with conventional distal gastrectomy. Surgery 1998;123:165-70. [Crossref] [PubMed]
- Zhang D, Shimoyama S, Kaminishi M. Feasibility of pylorus-preserving gastrectomy with a wider scope of lymphadenectomy. Arch Surg 1998;133:993-7. [Crossref] [PubMed]
- Hotta T, Taniguchi K, Kobayashi Y, et al. Postoperative evaluation of pylorus-preserving procedures compared with conventional distal gastrectomy for early gastric cancer. Surg Today 2001;31:774-9. [Crossref] [PubMed]
- Shibata C, Shiiba KI, Funayama Y, et al. Outcomes after pylorus-preserving gastrectomy for early gastric cancer: a prospective multicenter trial. World J Surg 2004;28:857-61. [Crossref] [PubMed]
- Urushihara T, Sumimoto K, Shimokado K, et al. Gastric motility after laparoscopically assisted distal gastrectomy, with or without preservation of the pylorus, for early gastric cancer, as assessed by digital dynamic x-ray imaging. Surg Endosc 2004;18:964-8. [Crossref] [PubMed]
- Nomura T, Fukushima N, Takasu N, et al. Evaluation of Pylorus Preserving Gastrectomy Compared with Conventional Distal Gastrectomy. Nippon Shokaki Geka Gakkai Zasshi 2005;38:1785-94. [Crossref]
- Hu X, Cao L, Yu Y, et al. Zhonghua Wei Chang Wai Ke Za Zhi 2010;13:907-9. [Efficacy observation after pylorus-preserving gastrectomy for early gastric cancer]. [PubMed]
- Ikeguchi M, Hatada T, Yamamoto M, et al. Evaluation of a pylorus-preserving gastrectomy for patients preoperatively diagnosed with early gastric cancer located in the middle third of the stomach. Surg Today 2010;40:228-33. [Crossref] [PubMed]
- Ikeguchi M, Kuroda H, Kihara K, et al. Nutritional assessment of patients after pylorus-preserving gastrectomy for early gastric cancer. Indian J Surg 2010;72:453-7. [Crossref] [PubMed]
- Lee SW, Nomura E, Bouras G, et al. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 2010;211:33-40. [Crossref] [PubMed]
- Tomikawa M, Korenaga D, Akahoshi T, et al. Quality of life after laparoscopy-assisted pylorus-preserving gastrectomy: an evaluation using a questionnaire mailed to the patients. Surg Today 2012;42:625-32. [Crossref] [PubMed]
- Suh YS, Han DS, Kong SH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg 2014;259:485-93. [Crossref] [PubMed]
- Hiki N, Nunobe S, Kubota T, et al. Function-preserving gastrectomy for early gastric cancer. Ann Surg Oncol 2013;20:2683-92. [Crossref] [PubMed]
- Nakajima T, Yamaguchi T. Gastric Cancer Data Base in Cancer Institute, Japan; 1946–2004. Tokyo: Kanehara & Co, Ltd 2006.
- Kong SH, Kim JW, Lee HJ, et al. The safety of the dissection of lymph node stations 5 and 6 in pylorus-preserving gastrectomy. Ann Surg Oncol 2009;16:3252-8. [Crossref] [PubMed]
- Ando S, Tsuji H. Surgical technique of vagus nerve-preserving gastrectomy with D2 lymphadenectomy for gastric cancer. ANZ J Surg 2008;78:172-6. [Crossref] [PubMed]
- Sano T, Sasako M, Kinoshita T, et al. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993;72:3174-8. [Crossref] [PubMed]
- Hiki N, Sano T, Fukunaga T, et al. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg 2009;209:297-301. [Crossref] [PubMed]
- Song P, Lu M, Pu F, et al. Meta-analysis of pylorus-preserving gastrectomy for middle-third early gastric cancer. J Laparoendosc Adv Surg Tech A 2014;24:718-27. [Crossref] [PubMed]
Cite this article as: Chen T, Chen Z, Zhen L, Zhong J, Wang X, Xu L, Qi X. Pylorus-preserving gastrectomy versus distal gastrectomy in oncology for the patient with early gastric cancer: a meta-analysis. AME Med J 2017;2:68.