ABCG2 rs2231142 polymorphism is related to sunitinib-induced toxicity in metastatic renal cell carcinoma: a systematic review
Introduction
Renal cell cancer (RCC) is a common cancer in the urinary system and accounts for nearly 2–3% of all malignancies (1). The estimated new cases in the United States in 2017 is 63,990, and estimated death is 14,400 (2). Besides, approximately a third of RCC patients suffering nephrectomy will progress to metastases renal cell cancer (mRCC) within 5 years (3). However, mRCC has been demonstrated to be insensitive to chemotherapy and hormone therapy (4,5). Additionally, immunotherapy (interleukin 2 and interferon, etc.) contributes limited effects to mRCC, and duration of action is transient (6).
With the exploration of molecular mechanisms of tumorigenesis and progression, targeted therapy came to prominence in the treatment of mRCC. Sunitinib, as a small-molecule receptor tyrosine kinase inhibitor (TKI), was approved by the Food and Drug Administration (FDA) in 2006. And it is now considered as a first-line therapy of mRCC (1) contributed by the direct anti-tumor and anti-angiogenic activity (7). Although sunitinib exposure increase the overall survival (OS), several common sunitinib-induced adverse events were frequently observed (8-11). A randomized, double-blind, phase 3 trial suggested that treatment discontinuations owing to adverse events occurred in 86 patients (28.1%) in the sunitinib group and 17 (5.6%) in the placebo group (12). Furthermore, studies have confirmed that single nucleotide polymorphisms (SNPs) might be associated with pharmacokinetics and pharmacodynamics of sunitinib, thus affecting the toxicity and efficacy to the patients. Moreover, it was observed that SNPs might have effects on progression-free survival (PFS) of mRCC (11,13) after sunitinib therapy. However, the conclusions were inconsistent and conflicting findings were observed (14).
The human ATP-binding cassette sub-family G member 2 (ABCG2) gene is located on chromosome 4q22 and encodes a 655 amino acid polypeptide (15). ABCG2 contains 6 transmembrane domains and an ATP-binding domain, and it acts as a half transporter member in the ABCG subfamily (16). From a perspective of tissue distribution, studies have shown that ABCG2 is over-expressed in human placenta, small intestine, colon, and the bile canalicular membrane (17). The specific localization of ABCG2 suggested that it might regulate intestinal absorption and biliary secretion of potentially toxic xenobiotics through active transport mechanisms (18,19). Moreover, investigations have reported that ABCG2 could mediate the efflux of various anti-cancer drugs, including sunitinib (20) and relevant sunitinib-induced toxicity (9). However, some researchers held opposite opinions (9,10). Accordingly, we performed such systematic review for more precise results and more comprehensive understanding of association between rs2231142 and sunitinib-induced toxicity.
Methods
Search strategy
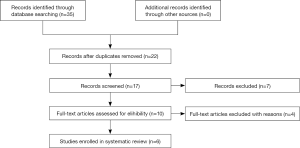
We performed a systematic literature search based on the ‘PRISMA’ guideline (21). A systematic search on PubMed, EMBASE and Web of Science was performed to identify all potentially appropriate studies (till March 31st, 2017). The following key words were utilized (“genetic polymorphism”, “SNP”, or “single nucleotide polymorphism”), (“metastases renal cell carcinoma”, “tumor”, or “cancer”), and (“rs2231142”, “ABCG2” and “ATP-binding cassette sub-family G member 2”). Additional publications were manually identified when we searched the reference lists of original articles. A flow diagram of the study selection process is presented in Figure 1.
Inclusion criteria and exclusion criteria
We filtered the articles with the following inclusion criteria: (I) studies estimating the associations between rs2231142 and sunitinib-induced toxicity in Mrcc; (II) data involved in different studies were not overlapping. Conversely, the exclusion criteria were exhibited as follows: (I) Studies consisted no usable data of sunitinib-induced toxicity in mRCC; (II) studies had overlapped data.
Data extraction
All useful information involved in eligible studies were extracted by two investigators (YZ and CZ) independently. The review of results was carried out by a third investigator (CM). Name of first author, year of publication, ethnicity, number of patients, basic information of total patients, sunitinib-induced toxicity and Eastern Cooperative Oncology Group (ECOG) score were extracted for each selected study. Besides, relevant data of association between rs2231142 and sunitinib-induced toxicity was extracted from multivariate logistic regression analysis in 6 enrolled studies. The selected adverse effects were thrombocytopenia, neutropenia, hand-foot syndrome, any toxicity higher than grade 2, diarrhea, fever, hypertension, hypothyroidism, leucopenia and proteinuria. Furthermore, Newcastle-Ottawa Scale was performed to evaluated the quality of all studies enrolled in our study.
Results
Six relevant studies (9-11,14,22,23) were finally included in our review (Table 1). A total of 698 patients (495 male, 203 female) with an average age of 61.31 years old (18–87 years old) were included. Among these studies, the white were mainly studied in 2 articles, and they accounted for more than 90% of the total enrolled patients. Other 4 studies mainly contained Asian patients. In addition, the detailed information of weight (2 studies) and BSA (4 studies) were shown in Table 1. Besides, 2 studies reported the RCC histology, and 5 reported ECOG score of patients. Newcastle-Ottawa Scale of each studies enrolled in our study were shown in Table 1.
Table 1
| First author | Year | Ethnicity | No. of patients | Age# (years) | Sex | Weight* | BSA* | mRCC Histology | ECOG score | NOS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | CCC | Other | Unknown | 0 | 1 | 2 | 3 | M | ||||||||||
| Garcia-Donas | 2011 | White | 95 | 65 [42–87] | 65 | 30 | NA | NA | NA | NA | NA | 25 | 56 | 8 | 0 | 6 | 6 | ||
| Kim | 2013 | Asian | 65 | 59 [36–81] | 51 | 14 | 65.3±0.03 | 1.7±0.1 | 61 | 3 | 1 | 18 | 37 | 8 | 1 | NA | 6 | ||
| Chu | 2015 | Asian | 97 | 58 [18–79] | 75 | 22 | NA | 1.63 [1.18–1.92] | NA | NA | N | 27 | 51 | 13 | 6 | NA | 7 | ||
| Low | 2016 | Asian | 219 | 63 [32–83] | 161 | 58 | NA | NA | 176 | 30 | 13 | 159 | 45 | 11 | 2 | 2 | 7 | ||
| Mizuno | 2012 | Asian | 19 | 62 [21–79] | 14 | 5 | 58 [44–78] | 1.59 [1.31–1.81] | NA | NA | NA | NA | NA | NA | NA | NA | 6 | ||
| van Erp | 2009 | White | 203 | 60 [20–84] | 129 | 74 | NA | 1.93 [1.47–2.51] | NA | NA | NA | 81 | 90 | 17 | 8 | 7 | 7 | ||
#, median age at time of sunitinib treatment [range]; *, mean ± SD or median [range]. CCC, clear cell carcinoma; mRCC, metastatic renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group; M, missing; NA, not available; NOS, Newcastle-Ottawa Scale.
In articles enrolled our study, the treatment plan (dose and schedule) for sunitinib was 50 mg each day given orally for 4 consecutive weeks followed by 2 weeks-off per treatment cycle. Data about toxicity of sunitinib extracted from enrolled studies was recorded during the first treatment cycle. Sunitinib toxicity was assessed with the National Cancer Institute-Common Toxicity Criteria for Adverse Effects (NCI-CTCAE) version 3.0. Afterwards, the results of association between rs2231142 and sunitinib-induced toxicity was summarized in Table 2. Studies from Kim and Low reported ABCG2 AA was related to thrombocytopenia (P=0.04, P<0.01, respectively). However, van Erp et al. suggested that rs2231142 was not associated with thrombocytopenia (P=0.12, respectively). Furthermore, rs2231142 was connected with neutropenia according to the results from the investigation of Kim and Chu (P=0.02, P=0.03, respectively), but the connection was not observed in Low’s study (P=0.37). Articles from Kim and Garcia-Donas revealed rs2231142 had considerable association with hand-foot syndrome (P=0.01, P=0.04, respectively). On the contrary, the consequence was not shown in Low’s study (P=0.37). Kim investigated that rs2231142 was related to any hematologic toxicity > grade 2 (P=0.05). In addition, research from Low suggested that rs2231142 was connected to fever (P=0.02), but not relevant with diarrhea (P=0.31), hypertension (P=0.46), hypothyroidism (P=0.15), leucopenia (P=0.28) or proteinuria (P=0.21).
Table 2
| Sunitinib-induced toxicities | Genotype | OR | 95% CI | P value | First author |
|---|---|---|---|---|---|
| Thrombocytopenia | CC + CA; AA | 9.9 | (1.16–infinity) | 0.04 | Hye Ryun Kim |
| CC; CA + AA | 1.93 | (0.85–4.42) | 0.118 | Nielka P. van Erp | |
| CC + CA; AA | 1.856 | (1.172–2.939) | 0.000841 | Siew-Kee Low | |
| Neutropenia | CC + CA; AA | 18.2 | (1.49–222.09) | 0.02 | Hye Ryun Kim |
| CC + CA; AA | 0.3 | (0.1–0.9) | 0.03 | Ying-Hsia Chu | |
| CC + CA; AA | 0.856 | (0.512–1.431) | 0.553 | Siew-Kee Low | |
| Hand-foot syndrome | CC + CA; AA | 28.46 | (2.22–364.94) | 0.01 | Hye Ryun Kim |
| CC + CA; AA | 1.24 | (0.78–1.98) | 0.369 | Siew-Kee Low | |
| CC + CA; AA | 0.11 (HR) | (0.01–0.92) | 0.04 | Jesus Garcia-Donas | |
| Any hematologic toxicity > grade 2 | CC + CA; AA | 8.24 | (0.99–infinity) | 0.05 | Hye Ryun Kim |
| Diarrhea | CC + CA; AA | 1.468 | (0.703–3.067) | 0.307 | Siew-Kee Low |
| Fever | CC + CA; AA | 2.845 | (1.216–6.657) | 0.0159 | Siew-Kee Low |
| Hypertension | CC + CA; AA | 1.23 | (0.713–2.724) | 0.457 | Siew-Kee Low |
| Hypothyroidism | CC + CA; AA | 1.402 | (0.89–2.208) | 0.145 | Siew-Kee Low |
| Leucopenia | CC + CA; AA | 0.712 | (0.385–1.316) | 0.278 | Siew-Kee Low |
| Proteinuria | CC + CA; AA | 1.456 | (0.806–2.631) | 0.213 | Siew-Kee Low |
mRCC, metastatic renal cell carcinoma; OR, odds ratio; CI, confidence interval.
Discussion
Sunitinib is a multitargeted TKI for the treatment of mRCC. Following administration, sunitinib is primarily metabolized by cytochrome P450 3A4 to an active N-desethyl metabolite (SU12262) (24). In addition, ABCB2 plays a role of a half transporter of sunitinib, and acts as a homodimeric/oligomeric efflux pump (25). For this reason, the expression level of ABCG2 affects the metabolic of sunitinib, which leads to affects the exposure of sunitinib in vivo. For example, a study reported that brain accumulation of sunitinib was markedly (23-fold) increased in ABCB1-ABCG2 co-knockout mice, but only slightly (2.3-fold) in ABCB1 knockout mice (26). Thus, the exposure of sunitinib but not the dose should be the first condition to consider when we explore the toxicities caused by sunitinib. A meta-analysis enrolled 14 studies with 590 subjects suggested that increased exposure to sunitinib and/or SU12662, which may in turn increase the incidence or severity of adverse events (8).
Three variant ABCG2 cDNAs harboring the following substitutions: 34G > A (V12M), 421C > A (Q141K) and an amino acid deletion of residues 944-949 that lacks Ala-315 and Thr-316 (Δ315-6) (27). Besides, study suggested that rs2231142 ABCG2-transfected murine fibroblast PA317 (PA/Q141K) cells display lower exogenous ABCG2 protein levels than the wild-type ABCG2-transfected cells. Furthermore, in PA317 cells, intracellular topotecan accumulation was higher compared with other ABCG2 transfectants, showing rs2231142 might influence ABCG2 function (27). Additionally, rs2231142 was demonstrated to locate within the ATP-binding region between the Walker A and B motifs of ABCG2, and this polymorphism could regulate the activity of ATPase, which leaded to affect the biochemical process of cells (28,29). In summary, rs2231142 may affect the activity of ABCG2 as a sunitinib transport pump by altering the activity of intracellular ATPase and/or regulate the ABCB2 protein level, which allows sunitinib to accumulate in cells and the level of sunitinib exposure after administration, and enhance sunitinib-induced toxicity.
In our study, we found that rs2231142 may associated with sunitinib-induced toxicity such as thrombocytopenia, neutropenia, hand-foot syndrome, fever and any hematologic toxicity (> grade 2). However, the connections between rs2231142 and diarrhea, hypertension, hypothyroidism, leucopenia, proteinuria were not observed in our review.
Reports indicated that ABCG2 rs2231142 appeared to be a very common ABCG2 polymorphism in Asian populations, and the reported allelic frequencies were 27–34% (27,29,30). However, the frequency was approximately 10% in white populations, and less than 5% in sub-Sahara African and in African-American. Differences in SNP distribution among races may lead to the discrepancy of sunitinib-induced toxicity incidence in patients with different ethnicity. In our review, the adverse effects after sunitinib was mainly observed in studies enrolled Asian patients. However, several conflict results were exhibited in different Asian studies. To reduce the false positive rate, high-quality studies with large amount of samples are still needed, whether they are based on Asian populations or other races.
Limitations also existed in our review. First, we only enrolled 6 articles which focused on the association between rs2231142 and sunitinib-induced toxicity in mRCC. With a growing number of high-quality studies being published, more capable studies will be enrolled and summarized in the future. Second, our systematic review was based on individual articles with different contents, which may reduce the representation of the final results. Third, more indicators about sunitinib-induced toxicity have yet to be further explored, and further confirmation with long term results were required.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.24). Dr. Li serves as an unpaid section editor of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Leibovich BC, Han KR, Bui MH, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;98:2566-75. [Crossref] [PubMed]
- Poster DS, Bruno S, Penta JS, et al. Current status of chemotherapy, hormonal therapy, and immunotherapy in the treatment of renal cell carcinoma. Am J Clin Oncol 1982;5:53-60. [Crossref] [PubMed]
- Harris DT. Hormonal therapy and chemotherapy of renal-cell carcinoma. Semin Oncol 1983;10:422-30. [PubMed]
- Liu KG, Gupta S, Goel S. Immunotherapy: incorporation in the evolving paradigm of renal cancer management and future prospects. Oncotarget 2017;8:17313-27. [PubMed]
- Vakkalanka BK, Bukowski RM. Novel drugs for renal cell carcinoma. Expert Opin Investig Drugs 2008;17:1501-16. [Crossref] [PubMed]
- Houk BE, Bello CL, Kang D, et al. A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 2009;15:2497-506. [Crossref] [PubMed]
- Kim HR, Park HS, Kwon WS, et al. Pharmacogenetic determinants associated with sunitinib-induced toxicity and ethnic difference in Korean metastatic renal cell carcinoma patients. Cancer Chemother Pharmacol 2013;72:825-35. [Crossref] [PubMed]
- Low SK, Fukunaga K, Takahashi A, et al. Association Study of a Functional Variant on ABCG2 Gene with Sunitinib-Induced Severe Adverse Drug Reaction. PLoS One 2016;11:e0148177. [Crossref] [PubMed]
- van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol 2009;27:4406-12. [Crossref] [PubMed]
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375:2246-54. [Crossref] [PubMed]
- van der Veldt AA, Eechoute K, Gelderblom H, et al. Genetic polymorphisms associated with a prolonged progression-free survival in patients with metastatic renal cell cancer treated with sunitinib. Clin Cancer Res 2011;17:620-9. [Crossref] [PubMed]
- Garcia-Donas J, Esteban E, Leandro-Garcia LJ, et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: a multicentre, observational, prospective study. Lancet Oncol 2011;12:1143-50. [Crossref] [PubMed]
- Allikmets R, Schriml LM, Hutchinson A, et al. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998;58:5337-9. [PubMed]
- Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2). Mol Cancer Ther 2002;1:427-34. [PubMed]
- Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 2001;61:3458-64. [PubMed]
- Jonker JW, Buitelaar M, Wagenaar E, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci USA 2002;99:15649-54. [Crossref] [PubMed]
- Jonker JW, Smit JW, Brinkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst 2000;92:1651-6. [Crossref] [PubMed]
- Noguchi K, Katayama K, Mitsuhashi J, et al. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev 2009;61:26-33. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Chu YH, Li H, Tan HS, et al. Association of ABCB1 and FLT3 Polymorphisms with Toxicities and Survival in Asian Patients Receiving Sunitinib for Renal Cell Carcinoma. PLoS One 2015;10:e0134102. [Crossref] [PubMed]
- Mizuno T, Fukudo M, Terada T, et al. Impact of genetic variation in breast cancer resistance protein (BCRP/ABCG2) on sunitinib pharmacokinetics. Drug Metab Pharmacokinet 2012;27:631-9. [Crossref] [PubMed]
- Noé G, Bellesoeur A, Thomas-Schoemann A, et al. Clinical and kinomic analysis identifies peripheral blood mononuclear cells as a potential pharmacodynamic biomarker in metastatic renal cell carcinoma patients treated with sunitinib. Oncotarget 2016;7:67507-20. [PubMed]
- Bhatia A, Schafer HJ, Hrycyna CA. Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry 2005;44:10893-904. [Crossref] [PubMed]
- Tang SC, Lagas JS, Lankheet NA, et al. Brain accumulation of sunitinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by oral elacridar and sunitinib coadministration. Int J Cancer 2012;130:223-33. [Crossref] [PubMed]
- Imai Y, Nakane M, Kage K, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 2002;1:611-6. [PubMed]
- Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer 2004;109:238-46. [Crossref] [PubMed]
- Kondo C, Suzuki H, Itoda M, et al. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res 2004;21:1895-903. [Crossref] [PubMed]
- Itoda M, Saito Y, Shirao K, et al. Eight novel single nucleotide polymorphisms in ABCG2/BCRP in Japanese cancer patients administered irinotacan. Drug Metab Pharmacokinet 2003;18:212-7. [Crossref] [PubMed]
Cite this article as: Zheng Y, Zhang C, Miao C, Lu P, Ma B, Si S, Xu W, Wang J, Li X. ABCG2 rs2231142 polymorphism is related to sunitinib-induced toxicity in metastatic renal cell carcinoma: a systematic review. AME Med J 2017;2:78.