Our gut bacteria may act as a foe of our brain
Fatty acids, the simplest form of lipids, are basic building blocks of our life. With their various structure diversity (such as the length of carbon chain, numbers of double bonds, cis-trans forms and other functional residues on the main carbon chain, for example hydroxyl or methyl groups), they are expected to perform various unique and critical functions in biological process. Otherwise those structural diversity would be gradually lost during the billions years of natural selection (1). Unfortunately, our knowledges about fatty acid functions are still very limited. It is important to know that except our own cells and food uptake, the gastrointestinal bacteria residing in our body can also produce a lot of fatty acids during fermentation processes, in particular, acetate, propionate, and butyrate (2). The effects of those SCFAs were not attracting much attending until recent several exciting discoveries (3).
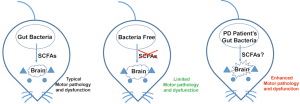
In a recent published paper, Sampson et al., reported that gut microbes could promote motor dysfunction in an alpha-synuclein-overexpressing Parkinson’s disease (PD) mouse model (ASO mice) (4) (Figure 1). Comparing to the antibiotic-treated specific pathogen-free (SPF) ASO mice, Sampson et al., found germ free (GF) ASO mice showed reduced motor deficits. Indicating intestinal bacteria might play a role in the pathogenesis. They further found GF-ASO mice displayed fewer alpha-synuclein aggregates, probably by increasing the surrounding microglia dependent alpha-synuclein-aggregates-clearance process during the postnatal stage (4).
The most interesting finding by Sampson et al., showed that the potential cause of those motor dysfunction in SPF-ASO mice is SCFAs, a class of fermentation metabolites generated by intestinal microbiota (4). SCFAs previously have also been shown to modulate microglia activation (5). Therefore, the authors hypothesized that SCFAs could also be the same reason to affect alpha-synuclein-aggregates-clearance via modulating microglia. To prove their hypothesis, they simply fed GF-ASO mice with a mixture of SCFAs including acetate, propionate and butyrate. The SCFA-fed GF-ASO mice surprisingly recapitulated similar microglia morphology changes, increasing alpha-synuclein aggregates and motor deficits, just like they have gut bacteria within(4).
These authors did not just restrict their finding in mice. They also evaluated whether gut bacteria collected from human PD patients could enhance similar motor deficits in GF-ASO mice. Their results were surprising: GF-ASO recipient mice showed decreased motor ability when given fecal bacteria from PD patients. By microbiota genome analyses, they found the abundance of certain bacteria species (Such as Proteus sp., Bilophila sp., and Roseburia sp.) were increased in gut microbiota in PD patients (4). The abundance of SCFAs producing enzymes (such as butyrate kinase and acetate CoA/acetoacetate CoA transferase alpha and beta) were also increased in mice recipient transplanted PD patients’ gut bacteria. Therefore, Sampson et al., suggested that gut microbiota might also enhance PD symptoms in human patients (4).
While the authors made extremely interesting and significant findings, several questions were not answered in this article. First, the authors did not explain why under SCFAs feeding, the enhanced a-synuclein aggregates and brain morphology changes happened in different brain regions of GF-ASO mice. It was also not known why abundance of acetate was decreased in PD donor-derived microbiota, but mixture SCFAs including acetates could promote the motor deficits in GF-ASO mice. The authors implied that butyrate might be the real cause. However, the butyrate concentrations in gut bacteria from health people were 50% higher than from SPF-ASO mice, yet it did not enhance the motor deficit in GF-ASO. Finally, the authors found that SCFAs could not cause a-synuclein aggregate in vitro (4). All these potential problems could not be addressed, until the critical questions have been addressed: which SCFA, and how it affects the a-synuclein aggregates in neurons in vivo. The authors mentioned known SCFAs receptor such has FFAR2 is not expressed in related brain regions, therefore this pathway is not likely involved (4). Future works on this field could be very helpful to increase the understanding of the pathological mechanism of SCFAs in PDs. For example, how SCFAs have been metabolized in intestine or in neuron/microglia? How they or their metabolites are transported into the neuron/microglia cells? Do SCFAs or SCFAs metabolites in neuron/microglia cells function with other organelles/proteins to regulated clearance of a-synuclein aggregates?
Gut bacteria produced SCFAs play many significant roles in human health in addition to neuronal degenerative diseases. SCFAs were found function as histone deacetylases inhibitor and ligands for G-protein coupled receptors (GPCR) (3,6). SCFAs supplement were also reported beneficial to human health such as obesity resistance and improved glucose homeostasis (7). Moreover, the drug-independent therapeutic method (fecal transplant instead) might also be very attractive to many diseases (Though an investigational new drug permit is still required by FDA). Taken together, despite the research challenges mentioned above, functional and mechanistic studies of gut SCFAs produced by gut bacteria could be a potential uprising field and worth more attention.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Ziwei Li (Department of Molecular, Cell & Developmental Biology, University of California, Los Angeles, USA).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.19). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu H, Han M. Exploring developmental and physiological functions of fatty acid and lipid variants through worm and fly genetics. Annu Rev Genet 2014;48:119-48. [Crossref] [PubMed]
- Cummings JH, Pomare EW, Branch WJ, et al. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987;28:1221-7. [Crossref] [PubMed]
- Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165:1332-45. [Crossref] [PubMed]
- Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell 2016;167:1469-80 e12.
- Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965-77. [Crossref] [PubMed]
- Flint HJ, Scott KP, Louis P, et al. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012;9:577-89. [Crossref] [PubMed]
- Venter CS, Vorster HH, Cummings JH. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am J Gastroenterol 1990;85:549-53. [PubMed]
Cite this article as: Zhu H. Our gut bacteria may act as a foe of our brain. AME Med J 2017;2:87.