
Approach to thrombophilia testing in patients with splanchnic vein thrombosis
Introduction
Thrombosis of the splanchnic vasculature is an uncommon but potentially lethal condition (1). Splanchnic vein thrombosis is a term that encompasses thrombosis in any of the mesenteric, splenic, and portal veins. Thrombosis of the hepatic veins, also known as Budd-Chiari syndrome, is also a type of splanchnic vein thrombosis but appears to have different risk factors and will not be addressed. Given the anatomic merging of the superior mesenteric and splenic veins to form the portal vein, these three are usually thought of when the term “splanchnic vein thrombosis” is used. Similarly, Zarrouk and colleagues define mesenteric venous thrombosis (MVT) as thrombosis of the superior mesenteric vein in isolation or with the splenic or portal veins (2). The authors present a retrospective review of their center’s experience with MVT and a systematic review of the literature focused on the evaluation for and prevalence of risk factors for MVT. They demonstrated in both their institutional cohort and the systematic review that the highest frequency risk factor was any thrombophilia, with slight differences between their cohort and the systematic review. Both groups found that the factor V Leiden (FVL) mutation was the most frequent risk factor, with a history of venous thromboembolism (VTE) the second most frequent. The inherited FVL and prothrombin G20210 gene mutations as well as the acquired JAK2 V617F mutation were more frequently found in patients with MVT in both in the authors’ population and the systematic review than in the general population. Large, recent studies of patients with splanchnic vein thrombosis demonstrate that a majority of patients have underlying local or systemic provoking risk factors, underscoring the importance of this evaluation (3,4). We present an algorithm for assessing splanchnic vein thrombosis risk factors, including local and systemic risks as well as thrombophilia.
Each of the four subtypes of splanchnic vein thrombosis (mesenteric, portal, splenic, and multiple vein) has different associations with the various thrombophilias, suggesting possible differences in underlying pathophysiology. Therefore, studies examining thrombophilia prevalence in these entities should consider them separately (5,6). The original retrospective cohort presented by Zarrouk and colleagues does not do this, combining all cases of isolated MVT and multiple vein thrombosis and referring to all as cases of MVT without distinction. Although the objective of the systematic analysis was examination of the prevalence of thrombophilias in patients with MVT, the analysis included studies of patients with all types of splanchnic vein thrombosis (such as isolated portal or splenic vein thrombosis) without excluding data from those other types of splanchnic thromboses. The result is an analysis of thrombophilia in MVT contaminated by many patients without MVT as defined by the authors’ stated definition. In addition, at least one study with patients with Budd-Chiari syndrome was included in the analysis, despite the authors’ intention to exclude this condition as well (2). We feel this is a significant weakness of the authors’ otherwise well-done systematic analysis. We consider the different subtypes of splanchnic vein thrombosis as individual entities because the existing body of data, though modest, suggests meaningful differences in the prevalence of each of the major inherited and acquired thrombophilias based on anatomic site of thrombosis within the splanchnic vasculature. We will review these differences.
Prior to evaluation for heritable or acquired thrombophilias in a patient with splanchnic vein thrombosis, a search for local compressive, inflammatory, or stasis-inducing risk factors should be performed. Local inflammatory factors that may precipitate splanchnic vein thrombosis include inflammatory bowel disease, acute pancreatitis, diverticulitis, intra-abdominal surgeries, and endoscopic procedures, such as sclerotherapy for the management of esophageal varices (7,8). Local compressive pathology that should be ruled out on imaging includes mesenteric lymphadenopathy, organomegaly, and compressive abdominal masses (9). Stasis of splanchnic venous blood flow is typically due to cirrhosis of any etiology and resultant portal hypertension or right heart failure (10). We advise a complete investigation for these factors in any patient presenting with splanchnic vein thrombosis; such factors can typically be confirmed or ruled out with a comprehensive history, physical exam, and the abdominopelvic cross-sectional imaging usually already obtained when diagnosing the splanchnic vein thrombosis. Other important predisposing factors for VTE of any location that should be considered in all patients with splanchnic vein thrombosis include personal or family history of VTE, malignancy, antiphospholipid antibody syndrome, estrogen exposure (including pregnancy and the postpartum period), trauma, immobilization, and chronic inflammatory states (11). Our discussion here will focus on thrombophilias for which the utility of testing is less certain.
The decision to screen for inherited and acquired thrombophilias in patients presenting with splanchnic vein thrombosis is nuanced and controversial. The preponderance of data in patients with splanchnic vein thrombosis suggests a high incidence of underlying inherited thrombophilic states even in patients with predisposing local factors (3,6). Zarrouk and colleagues summarize the guidelines for thrombophilia screening published by several professional societies in their Table 2 (2). These guidelines lack consensus for inherited thrombophilia testing for general VTE. They variably recommend comprehensive testing, no testing, and limited testing under certain clinical situations; none are validated (11). Unsurprisingly, inherited thrombophilia testing practices vary dramatically, from testing nearly all VTE patients to near complete abstinence from testing due to the low likelihood it would affect clinical management, the high cost of testing, or both (12). Thrombosis location is an important consideration in the decision to test, with cerebral or splanchnic vein thrombosis more concerning for inherited thrombophilia than typical VTE (11).
Whom to test
Our approach to thrombophilia testing in patients with splanchnic vein thrombosis is outlined in Figure 1. The first step in workup is investigation for local precipitating factors. We also advise JAK2 V617F mutational analysis if the patient has erythrocytosis, leukocytosis, thrombocytosis, or splenomegaly. In a patient with such overt evidence of a myeloproliferative neoplasm (MPN) who is JAK2 V617F mutation negative, a complete evaluation for an underlying MPN should be performed. While there are other mutations associated with the Philadelphia-negative MPNs, including JAK2 exon 12 mutations and those in the CALR and MPL genes, the incidence of these mutations in patients with splanchnic vein thrombosis and no overt evidence of MPN is extremely rare (13). In contrast, the presence of JAK2 V617F-mutated clones in patients with splanchnic vein thrombosis in the absence of overt evidence of an MPN is a well-documented phenomenon; many of these patients go on to develop a clinically evident MPN over time (13). Patients presenting with splanchnic vein thrombosis for whom JAK2 V617F mutational analysis is unnecessary are those patients with local provoking factors, single-vessel involvement, and no clinical findings suggestive of MPN. The distinction between single and multiple vein involvement is made in this case because published data suggests that patients with multiple vein thrombosis have a higher likelihood of MPN than those with single vein thrombosis (6). For those patients with an identified local precipitating factor and unexplained cytopenias or evidence of hemolysis, flow cytometric testing to rule out the presence of concomitant paroxysmal nocturnal hemoglobinuria (PNH) should be considered (14). The final variable to assess in patients with clear local precipitating factors is that of hereditary thrombophilia testing. Because patients with single splanchnic vein thrombosis are more likely to have an underlying inherited thrombophilia on the basis of multiple large patient series, testing for inherited thrombophilias (including FVL, prothrombin G20210A mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency) is recommended in patients with local provoking factors and no evidence of MPN or PNH. It is reasonable to consider in such patients with local provoking factors and multiple vein thrombosis as well, though the yield is expected to be lower (5,6). Importantly, the utility of inherited thrombophilia testing in general is qualified by the individual clinical scenario; although it is clinically useful only in cases where positive test results would alter management, how to manage splanchnic vein thrombosis is not well defined. Information that can contribute to understanding the etiology may provide psychological benefit.
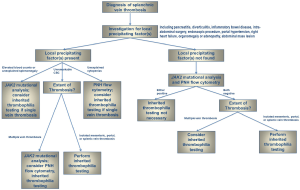
For patients without a clear local precipitating factor, the presence of an underlying thrombophilia is more likely, and a more comprehensive investigation is required. JAK2 V617F mutational analysis and flow cytometric testing for PNH should be performed in these patients. Any patient found to have a JAK2-mutated clone or significant PNH clone does not require further thrombophilia testing, as results will not affect management. If these tests are negative, inherited thrombophilia testing is the next consideration. We recommend screening for FVL, the prothrombin G20210A gene mutation, protein C deficiency, protein S deficiency, and antithrombin deficiency. This testing is most important for those patients with involvement of a single splanchnic vessel, as the likelihood of an underlying inherited thrombophilia is higher than in those with multiple vein thrombosis (5,6).
In the cohort presented by Zarrouk and colleagues of patients with MVT, testing for inherited thrombophilia revealed the following: FVL, 24%; prothrombin gene mutation, 3%; protein C deficiency, 2%; protein S deficiency, 6%; antithrombin deficiency, 0%. The JAK2 V617F mutation was found in 9% of patients. Interpretation of these results in the context of the existing series of patients with splanchnic vein thrombosis is difficult because the Zarrouk et al. study did not report separate incidences for isolated MVT and multiple vein thrombosis, instead combining all cases into a single study group. To contextualize the findings from this study, one can be compare them to the largest published investigation, by Sutkowska and colleagues, of thrombophilia prevalence to date in each subtype of splanchnic vein thrombosis (6). The prevalence of inherited thrombophilias, antiphospholipid antibodies, and MPNs from this 341-patient cohort (excluding 22 patients with Budd-Chiari syndrome) as well as prevalence of these disorders in the general population is given in Table 1. Both in this large series and in Zarrouk and colleagues’ study, FVL was substantially more prevalent in patients with MVT than in the general population, occurring in approximately one-quarter of cases. The prothrombin G20210A mutation was more common in MVT patients than the general population in the Sutkowska et al. study (9.1% versus 1–3%), but not the Zarrouk et al. study (only 3%). Of note, while neither of these studies found a major association of portal vein thrombosis with the prothrombin G20210A mutation, such an association has been seen in other studies, with a prevalence of 34.8% in one study of cirrhotic patients with portal vein thrombosis (16). The prevalence of protein C, protein S, and antithrombin deficiency in both series is variable, appearing to be higher than the general population on average but in overall numbers too low to draw significant conclusions. Importantly, MPNs were much more common in patients with multiple vein thrombosis (18%) versus MVT alone (1%), portal vein thrombosis alone (9%), or splenic vein thrombosis alone (0%) in the Sutkowska et al. study that examined these entities separately (6). Conversely, inherited thrombophilias were more common in patients with isolated MVT (48%) as opposed to multiple vein thrombosis (21.4%), a finding that has been noted in other studies (5). The major conclusions to draw from these studies are threefold: (I) inherited and acquired thrombophilias are very common in patients with splanchnic vein thrombosis; (II) the individual thrombophilia prevalence rates are influenced by the anatomic location of the splanchnic vein thrombosis; and (III) the differences in thrombophilia prevalence according to thrombosis location should help to guide the decision to test for each of the various thrombophilias and the sequence that such testing should follow to minimize unnecessary expense. Our algorithm for thrombophilia testing in patients with splanchnic vein thrombosis (Figure 1), based on our analysis of the best available evidence, can serve as a guide to testing.
Table 1
| Thrombophilia | MVT, % (n=67) | PVT, % (n=112) | SVT, % (n=11) | Multiple veins, % (n=129) | General population, % |
|---|---|---|---|---|---|
| Factor V Leiden | 25.4 | 4.5 | 27.3 | 7 | 3–7 |
| Prothrombin G20210A | 9.1 | 3.5 | 10.0 | 4.3 | 1–3 |
| Protein C deficiency | 0 | 0 | 0 | 0.8 | 0.2–0.4 |
| Protein S deficiency | 1.5 | 2.7 | 9.1 | 5.4 | 0.1–0.7 |
| Antithrombin deficiency | 3.0 | 1.8 | 0 | 0.8 | 0.02 |
| Antiphospholipid antibodies | 9.0 | 4.5 | 0 | 3.1 | 0–7 |
| Myeloproliferative neoplasm | 1.0 | 9.0 | 0 | 18.0 | – |
| Total | 49.0 | 26.0 | 46.4 | 39.4 | – |
MVT, mesenteric venous thrombosis; PVT, portal vein thrombosis; SVT, splenic vein thrombosis.
When to test
It is important to perform thrombophilia testing under proper conditions to minimize the likelihood of false-positive or false-negative testing results. While JAK2 mutational analysis and PNH flow cytometric testing may be performed at any time, several clot-based assays or specific protein levels performed as part of inherited thrombophilia testing can be influenced by anticoagulants and acute thrombosis. While genetic testing for FVL and the prothrombin G20210A mutation remain unaffected by these issues, protein C, protein S, and antithrombin assays may be, and testing is often obtained as a comprehensive panel. The outpatient setting (typically following a minimum of 3 months of anticoagulation), as opposed to the acute inpatient setting, is a more appropriate time to test, assuming anticoagulants can be held. Vitamin K antagonists must be held for at least two weeks and direct oral anticoagulants for at least 2–3 days. If there is strong concern for recurrent thrombosis when holding anticoagulation, test results are unlikely to modify management as the patient has already been deemed high-risk and will likely continue anticoagulation indefinitely (11). Testing at a later date if anticoagulation is to be stopped at that time could be considered in such a circumstance.
No data to support screening patients for inherited thrombophilia prior to surgeries known to have a high risk for splanchnic vein thrombosis are available. At least one study examined the utility of screening patients for inherited thrombophilia, lupus anticoagulant, and factor VIII level prior to laparoscopic splenectomy to discern if certain thrombophilias were associated with an increased risk of postoperative splenic or portal vein thrombosis. This study found no such associations, concluding that such preoperative screening was not useful to identify at-risk patients (17).
Conclusions
Presence of inherited or acquired thrombophilias is common in patients with splanchnic vein thrombosis. A rational approach to testing based on the best available evidence suggests examination for local precipitating factors in all patients, testing for JAK2 V617F in nearly all patients, and consideration of PNH screening, inherited thrombophilia testing, and antiphospholipid antibody testing in many patients. While in general testing should be performed only if it has the potential to modify clinical management in patients with non-splanchnic vein thrombosis, the development of splanchnic vein thrombosis can cause excessive anxiety and can be the result of underlying conditions for which knowing results could significantly alter management, such as with an MPN or PNH. Our algorithm suggests a stepwise approach to thrombophilia testing in this uncommon but high risk clinical entity.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.01.01). H Al-Samkari—Consultancy (Agios Pharmaceuticals). JM Connors—Scientific Advisory Board (Boehringer Ingelheim); Scientific Advisory Board, Consultant, Independent Review Committee (Bristol Meyer Squibb); Data Safety Monitoring Board (Unum Therapeutics).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rhee RY, Gloviczki P, Mendonca CT, et al. Mesenteric venous thrombosis: still a lethal disease in the 1990s. J Vasc Surg 1994;20:688-97. [Crossref] [PubMed]
- Zarrouk M, Salim S, Elf J, et al. Testing for thrombophilia in mesenteric venous thrombosis - Retrospective original study and systematic review. Best Pract Res Clin Gastroenterol 2017;31:39-48. [Crossref] [PubMed]
- Ageno W, Riva N, Schulman S, et al. Long-term Clinical Outcomes of Splanchnic Vein Thrombosis: Results of an International Registry. JAMA Intern Med 2015;175:1474-80. [Crossref] [PubMed]
- Thatipelli MR, McBane RD, Hodge DO, et al. Survival and recurrence in patients with splanchnic vein thromboses. Clin Gastroenterol Hepatol 2010;8:200-5. [Crossref] [PubMed]
- Kumar S, Kamath PS. Acute superior mesenteric venous thrombosis: one disease or two? Am J Gastroenterol 2003;98:1299-304. [Crossref] [PubMed]
- Sutkowska E, McBane RD, Tafur AJ, et al. Thrombophilia differences in splanchnic vein thrombosis and lower extremity deep venous thrombosis in North America. J Gastroenterol 2013;48:1111-8. [Crossref] [PubMed]
- Leach SD, Meier GH, Gusberg RJ. Endoscopic sclerotherapy: a risk factor for splanchnic venous thrombosis. J Vasc Surg 1989;10:9-12; discussion 12-3. [Crossref] [PubMed]
- Di Fabio F, Obrand D, Satin R, et al. Intra-abdominal venous and arterial thromboembolism in inflammatory bowel disease. Dis Colon Rectum 2009;52:336-42. [Crossref] [PubMed]
- Violi NV, Schoepfer AM, Fournier N, et al. Prevalence and clinical importance of mesenteric venous thrombosis in the Swiss Inflammatory Bowel Disease Cohort. AJR Am J Roentgenol 2014;203:62-9. [Crossref] [PubMed]
- Singal AK, Kamath PS, Tefferi A. Mesenteric venous thrombosis. Mayo Clin Proc 2013;88:285-94. [Crossref] [PubMed]
- Connors JM. Thrombophilia Testing and Venous Thrombosis. N Engl J Med 2017;377:1177-87. [Crossref] [PubMed]
- Cox N, Johnson SA, Vazquez S, et al. Patterns and Appropriateness of Thrombophilia Testing in an Academic Medical Center. J Hosp Med 2017;12:705-9. [Crossref] [PubMed]
- De Stefano V, Qi X, Betti S, et al. Splanchnic vein thrombosis and myeloproliferative neoplasms: molecular-driven diagnosis and long-term treatment. Thromb Haemost 2016;115:240-9. [Crossref] [PubMed]
- Ageno W, Dentali F, De Stefano V, et al. Clonal populations of hematopoietic cells with paroxysmal nocturnal hemoglobinuria phenotype in patients with splanchnic vein thrombosis. Thromb Res 2014;133:1052-5. [Crossref] [PubMed]
- Khan S, Dickerman JD. Hereditary thrombophilia. Thromb J 2006;4:15. [Crossref] [PubMed]
- Amitrano L, Brancaccio V, Guardascione MA, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology 2000;31:345-8. [Crossref] [PubMed]
- Manouchehri N, Kaneva P, Seguin C, et al. Screening for thrombophilia does not identify patients at risk of portal or splenic vein thrombosis following laparoscopic splenectomy. Surg Endosc 2016;30:2119-26. [Crossref] [PubMed]
Cite this article as: Al-Samkari H, Connors JM. Approach to thrombophilia testing in patients with splanchnic vein thrombosis. AME Med J 2018;3:7.

