
The technique of cortical bone trajectory screw fixation in spine surgery: a comprehensive literature review
Introduction
Spinal fusion with bilateral traditional pedicle screw (TPS) fixation has been described for various surgical indications, such as spinal degenerative disc disease, spinal canal stenosis, spondylolisthesis, spinal trauma, spinal tumor and deformity (1-5). However, TPS fixation has some drawbacks, including significant muscle dissection required for the exposure of anatomical bony landmarks. Although a percutaneous pedicle screws (PPS) technique can be alternative (6,7), it requires an additional approach for decompression and bone graft (8,9). Besides, the PPS technique depends on intraoperative multiplanar fluoroscopy, which results in high risk of radiation exposure of the surgeons and patients (10).
Additionally, screw loosening is a well-known complication of TPS fixation (11), especially in osteoporotic patients (12-14). Several methods can enhance screw purchase, such as modifying screw design, augmenting vertebral bodies with reinforcing materials or bicortical pedicle screw techniques (15-17). However, they still cannot be used in severe osteoporotic patients (18). Bone cement may associated with disadvantages such as its high exothermic polymerizing temperature, toxicity of the monomer, and risk of cement leakage to the spinal canal (19,20).
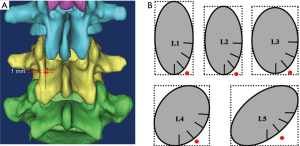
In 2009, Santoni et al. (18) introduced a novel method of pedicle screw insertion known the cortical bone trajectory (CBT). Used for lumbar screw that is shorter and smaller in diameter which has been proposed to maximize the thread contact with the zone of higher density bone density (21). CBT screw follows a medial-to-lateral path in the transverse plane and a caudal-to-cephalad path in the sagittal plane through the pedicle (Figure 1).

The CBT screw fixation can achieve four bone cortex sites: the dorsal, posteromedial, and anterolateral sides of the pedicle, and the lateral region of the vertebral body (22). Several cadaveric experiments demonstrated that CBT technique has equivalent or superior to the biomechanical properties than TPS (23,24). Moreover, the screw insertion point of this technique is located around the lateral portion of the pars interarticularis, offering advantages to avoid wide exposure of the superior facet joint and requiring less tissue dissection. In this study, we performed a comprehensive literature review of the history, development, biomechanical and clinical outcomes of CBT screw fixation technique.
Literature search methods
A systematic literature search of PubMed, EMBASE, OVID, Science Direct, and the Cochrane Collaboration data base was conducted through August 2017, based on the key words of “cortical bone trajectory”, “CBT”, “cortical trajectory”, “cortical screw”, “cortical trajectory screw”, “pedicle screw”, “osteoporosis”. The reference lists of all retrieved articles were reviewed to identify additional potentially relevant studies. Biomechanical, morphometric or clinical studies that reported complications, technique, efficacy, anatomy or animal or cadaveric studies on CBT screw fixation for spinal pathologies were included.
CBT screw fixation for lumbar spine
CBT screw starting point and parameters
Matsukawa et al. (22) proposed that the starting point of CBT for lumbar was located at the junction of the center of the superior articular process and 1 mm inferior to the inferior border of the transverse process, which was projected to the 5 o’clock orientation in the left pedicle and the 7 o’clock orientation in the right pedicle (Figure 2). They found the mean diameter gradually increased from L1 to L5 (from 6.2 mm at L1 to 8.4 mm at L5). The mean length from L1 to L5 was 36.8, 38.2, 39.3, 39.8, and 38.33 mm. The lateral angle and cephalad angle were 8°–9° and 25°–26°, and was not affected by the segment level. Chen et al. (25) measured the above data on Chinese population, with similar trends observed.
In the original CBT approach, it is necessary to expose the bone mark of inferior articular process. However, in many cases, this joint may have been destroyed or degenerated in many patients who have indications for intervertebral fusion, or have severe lateral slippage, potentially resulting in injury to the spinal canal and/or nerve root. Iwatsuki et al. (26) developed isthmus-guided CBT technique, and put the starting point at the lateral margin of the isthmus and superior margin of the intervertebral foramen (Figure 3). Although the isthmus-guided CBT technique can improve the accuracy of screw insertion, the shorter screw length and the fact that the starting point is closer to the upper endplate may result in reduced screw-bone contact.
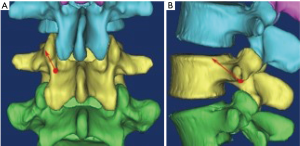
Senoglu et al. (27) evaluated 100 computed tomography (CT) scans of the lumbar spine and determined measurements of screw starting points, trajectories, and lengths of placement of CBT screws. They suggested that a pedicle-pars interarticularis junction length of less than 7.0 mm is too thin to safely accommodate a 5.0-mm screw given the preference for a minimum 1 mm bone stock on each side of the CBT screw. Based on the above theory, they found that the pedicle-pars interarticularis junction from L1 to L5 was deemed too small for 5 mm diameter CBT screw on the right 35%, 24%, 17%, 17%, and 19%, respectively, and on the left in 30%, 17%, 17%, 17%, and 20%, respectively. The average length of a screw placed along the cranial CBT measured 27–30.5 mm (±4.1°–6.2°). The parasagittal angle was ranging from 13°–16°.
The detailed parameters of lumbar CBT screw fixation are summarized in Table 1.
Table 1
| Items | Matsukawa et al., 2014 | Chen et al., 2015 | Senoglu et al., 2017 | ||||
|---|---|---|---|---|---|---|---|
| Total | Male | Female | Right side | Left side | |||
| No. of subjects | 100 | 80 | 80 | 100 | 100 | ||
| L1 | |||||||
| PH (mm) | 16.5±1.3 | 17.17±1.57 | 15.96±1.73 | – | – | ||
| PW (mm) | 7.9±1.5 | 9.00±1.71 | 6.76±1.56 | – | – | ||
| SD (mm) | 6.2±1.1 | 6.42±1.27 | 6.00±1.42 | – | – | ||
| SL (mm) | 36.8±3.2 | 36.17±2.38 | 35.57±2.21 | – | – | ||
| LA (°) | 8.6±2.3 | 8.28±2.39 | 8.63±2.21 | 13.94±3.45 | 14.13±3.02 | ||
| CA (°) | 26.2±4.5 | 26.77±3.45 | 26.69±4.20 | – | – | ||
| L2 | |||||||
| PH (mm) | 15.8±1.5 | 16.68±1.62 | 15.85±1.32 | – | – | ||
| PW (mm) | 8.0±1.4 | 8.42±1.49 | 7.53±1.33 | – | – | ||
| SD (mm) | 6.2±1.1 | 6.46±1.59 | 6.09±1.99 | – | – | ||
| SL (mm) | 38.2±3.0 | 36.63±2.42 | 36.17±2.72 | – | – | ||
| LA (°) | 8.5±2.4 | 9.81±2.67 | 9.29±3.88 | 13.5±2.75 | 13.45±2.6 | ||
| CA (°) | 25.5±4.5 | 26.27±3.45 | 25.01±3.93 | – | – | ||
| L3 | |||||||
| PH (mm) | 15.6±1.3 | 17.02±1.53 | 14.92±1.25 | – | – | ||
| PW (mm) | 9.6±1.6 | 10.03±1.79 | 8.37±1.52 | – | – | ||
| SD (mm) | 6.6±1.2 | 7.54±1.59 | 6.54±1.91 | – | – | ||
| SL (mm) | 39.3±3.3 | 38.22±2.25 | 37.05±2.59 | – | – | ||
| LA (°) | 9.1±2.4 | 9.33±2.25 | 9.49±2.28 | 13.62±2.92 | 13.0±2.34 | ||
| CA (°) | 26.2±4.9 | 26.25±2.89 | 26.40±2.38 | – | – | ||
| L4 | |||||||
| PH (mm) | 14.4±1.5 | 15.49±1.94 | 14.11±1.62 | – | – | ||
| PW (mm) | 11.3±1.7 | 13.23±2.06 | 10.11±1.62 | – | – | ||
| SD (mm) | 7.1±1.3 | 8.33±1.01 | 7.27±1.31 | – | – | ||
| SL (mm) | 39.8±3.5 | 37.85±2.19 | 37.08±2.64 | – | – | ||
| LA (°) | 9.1±2.3 | 9.77±1.55 | 9.51±2.09 | 13.89±3.03 | 14.11±2.77 | ||
| CA (°) | 26.0±4.4 | 26.27±2.14 | 26.65±2.48 | – | – | ||
| L5 | |||||||
| PH (mm) | 13.9±1.5 | 15.01±1.62 | 13.32±1.85 | – | – | ||
| PW (mm) | 15.3±2.0 | 15.47±2.36 | 14.33±1.99 | – | – | ||
| SD (mm) | 8.4±1.4 | 11.70±1.68 | 10.27±1.61 | – | – | ||
| SL (mm) | 38.3±3.9 | 37.88±2.30 | 36.76±2.71 | – | – | ||
| LA (°) | 8.8±2.1 | 9.41±1.23 | 9.45±1.34 | 15.57±3.67 | 15.22±3.53 | ||
| CA (°) | 25.8±4.8 | 27.63±2.68 | 26.25±2.70 | – | – | ||
CBT, cortical bone trajectory; CT, computed tomography; PH, pedicle height; PW, pedicle width; SD, screw diameter; SL, screw length; LA, lateral angle; CA, cephalad angle.
Biomechanical stability
Santoni et al. (18) found that CBT screws and TPSs have equivalent pullout strengths and toggle characteristics. CBT screws exhibit a 30% increase in uniaxial pullout strength relative to TPSs. However, screws for the traditional pedicle trajectory and CBT were different. Whether screw or trajectory affects the uniaxial pullout strength was unclear. Ueno et al. (28) investigated the relationship between screw entry trajectory or screw thread characteristics and pullout strength in pig cadaver experiments. The results showed that cortical screw could increase the fixation strength, but not significantly increase the pullout strength. The specific trajectory seemed to have a major impact on the pullout strength.
Calvert et al. (24) investigated the biomechanical properties of TPS and CBT screw when each was used to rescue the other in the setting of revision in ten fresh-frozen human lumbar spines. Data in this study showed that CBT rescue screws retained 60% of the original TPS pullout strength, whereas traditional rescue screws retained 65% of the original CBT screw pullout strength. It supported that either CBT or TPS use as a rescue option in the setting of a failed or compromised pedicle screw construct in the lumbar spine. Baluch et al. (29) found that the CBT screw had more resistance to loosening in fatigue testing when compared with the TPS. Perez-Orribo et al. (30) reported that there were no significant difference in the mean range of motion or lax zone of CBT screw and TPS during any loading mode. Matsukawa et al. (23) reported that CBT screws exhibit 2.01 greater insertional torque compared to TPSs in vivo.
Clinical outcomes
Ueno et al. (31) employed a double-trajectory technique (CBT combined with traditional trajectory) in a patient with degenerative lumbar scoliosis and severe osteoporosis. After 14 months follow-up evaluation, the patient’s postoperative clinical symptoms had been alleviated and there had been no loss of correction. Rodriguez et al. (32) utilized CBT screw fixation with intraoperative CT (O-arm) image-guided navigation to stabilize spinal levels in five consecutive patients with symptomatic adjacent-segment lumbar disease. After 10–15 months clinical follow-up, all patients reported improved symptoms from their preoperative state. Radiographic follow-up demonstrated Lenke fusion grades of A or B. Mizuno et al. (33) adopted midline lumbar fusion (MIDLF) technique, which is composed of posterior midline approach, microsurgical laminectomy, and CBT screw fixation, for treating 12 patients with single level of lumbar spondylolisthesis. One intraoperative complication was noted, which was cortical bone fracture at the screw compression. No patient had surgery-related spinal nerve injury or neurological deficit. After 20 months’ follow-up in five cases, there was no screw loosing or backout. Moreover, in 9 patients out of 12, the inflammatory markers data of CK and WBC recovered within a week, which was equivalent to the data of mini open PLIF in the literature. Takata et al. (34) performed hybrid reconstruction (CBT at the cranial level and TPS at the caudal level) on six patients with degenerative spondylolisthesis, the skin incision of above technique was around 5–6 cm, which was shorter than that of the TPS. Gonchar et al. (35) performed a prospective nonrandomized comparative clinical study of comparing outcomes of single-level MIS spinal fusion using CBT vs. PPS. At 6 months post-operation, results showed that single-level MIS posterior lumbar fusion with CBT screws had lower rate of screw loosening, less loss of correction, and was less invasive compared to that with PPS. Lee et al. (36) evaluated a prospective randomized non-inferiority trial of comparing clinical and radiological outcomes of CBT in PLIF and TPS in PLIF. According to the results, CBT provided similar fusion rates, VAS scores for lower back pain, and ODI scores, without significant differences. However, the occurrence of facet joint violation and surgical morbidities, including blood loss, operation time, hospital stay, and incision length was lower in CBT with PLIF group, compared that with TPS. Orita et al. (37) introduced a percutaneous CBT (pCBT) fixation technique by modifying the PPS technique and performed a prospective study of TLIF with pCBT or PPS on 40 patients. The results showed that pCBT group had advantages of shorter total incision length, shorter duration of fluoroscopy, compared with PPS group.
Glennie et al. (38) retrospective reviewed a series of eight patients using a CBT screw fixation for degenerative conditions of the lumbar spine. After an average of 12 months follow-up, four patients lost the maintenance of reduction, five patients had screw loosening, and two patients required revision surgery for pseudarthrosis and caudal adjacent segment failure. Pacione et al. (39) reported a case report of an 83-year-old woman patient with a combination of osteoporotic compression fracture and spinal stenosis, who underwent an L4/5 decompressive laminectomy, L4 kyphoplasty, and L3–5 instrumentation and fusion with CBT screw fixation. One month postoperatively, the patient had a new L3 compression fracture, and subsequently went a percutaneous parapedicular L3 kyphoplasty.
In addition to the stronger fixation strength, the CBT screw offers several other advantages over the TPS. Firstly, a lower risk of canal breach and subsequent neurologic injury given the medial to lateral and caudal to cephalad trajectory applied in the CBT technique. Secondly, CBT screw insertion through a more medial starting point enables a reduction in incision length, extent of muscle dissection, intraoperative retraction, and recovery time. Thirdly, the lateral trajectory through the pedicle reduces the risk of injury to the medial branch nerve that originated from the dorsal rami of each of the lumbar spinal nerves and thereby reduces the incidence of postoperative radiculitis (40). Moreover, contrary to PPS technique, another potential advantage using CBT technique is that all surgical procedures including laminectomy, interbody work, and screw placement are possible with limited midline exposure
CBT screw fixation for thoracic and sacral spine
Matsukawa et al. (41) investigated CBT technique in lower thoracic spine region (T9–T12), which was angulating cranially toward the posterior one-third of the superior endplate in the sagittal plane, and directed straight forward in the transverse plane. Morphometric measurement of thoracic CBT increased from T9 to T12 (the mean diameter: from 5.8 mm at T9 to 8.5 mm at T12, the length: from 29.7 mm at T9 to 32.0 mm at T12, and cephalad angle: from 21.4° at T9 to 27.6° at T12). In addition, the CBT technique demonstrated average 53.8% higher maximum insertional torque than the TPS (P<0.01).
Xuan et al. (42) evaluated the feasibility of CBT screw fixation via pedicle or pedicle rib unit in the lower thoracic spine (T9–T12). Maximal screw length obtained by CT has a tendency to gradually increase from T9 (29.64 mm) to T12 (32.84 mm). Maximal screw diameter increases from T9 (4.92 mm) to T12 (7.47 mm). Lateral angle increases from T9 (7.37°) to T12 (10.47°). Cephalad angle from T9 to T12 are 19.03°, 22.10°, 25.62° and 27.50°, respectively. In cadaveric thoracic experiment, the percentage of the inner and outer pedicle breakage are 2.5% and 22.5%, respectively. The violation of lateral pedicle wall occurs at T9 and T10, especially for women at T9. They also investigated the anatomical data and feasibility of performing 4.5 to 5.5 mm CBT screws fixation via pedicle or pedicle rib unit in the pediatric thoracic spine (T9–T12). Their studies supplied the additional evidence and novel pattern to CBT screws fixation in lower thoracic spine (43).
Sheng et al. (44) performed an anatomico-radiological study on the morphometrics of the middle-upper thoracic spine. The maximum length of the trajectory, the maximum diameter, and the cephalad angle exhibited a slight increase trend while the transverse and sagittal angles of the pedicle tended to decrease from T3 to T8. They recommended that the width of CBT screw for middle-upper thoracic spine is 5.0 mm, the length is 25 to 35 mm. The caudocephaled angles were 15° to 20°, and directed straight forward in the transverse plane. The detailed parameters of CBT screw fixation in thoracic region are summarized in Table 2.
Table 2
| Measurements | Matsukawa et al., 2014 (N=50) | Xuan et al., 2016 (N=100) | Sheng et al., 2016 (N=80) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD (mm) | SL (mm) | CA (°) | SD (mm) | SL (mm) | LA (°) | CA (°) | SD (mm) | SL (mm) | LA (°) | CA (°) | |||
| T9 | 5.8±1.1 | 29.7±4.6 | 21.4±3.3 | 4.92±0.64 | 29.64±0.94 | 7.37±1.39 | 19.03±2.68 | – | – | – | – | ||
| T10 | – | – | 24.6±3.0 | 5.83±0.86 | 30.79±1.45 | 8.58±2.25 | 22.10±2.67 | – | – | – | – | ||
| T11 | – | 32.0±2.1 | 26.9±2.9 | 6.88±1.10 | 31.64±1.34 | 10.14±2.69 | 25.62±3.09 | – | – | – | – | ||
| T12 | 8.5±1.4 | – | 27.6±3.9 | 7.47±1.08 | 32.84±1.82 | 10.47±2.90 | 27.50±3.63 | – | – | – | – | ||
| T3 | – | – | – | – | – | – | – | 3.61±0.46 | 23.63±1.96 | 3.61±0.46 | 18.77±1.83 | ||
| T4 | – | – | – | – | – | – | – | 3.88±0.41 | 25.44±1.88 | 3.88±0.41 | 19.20±1.25 | ||
| T5 | – | – | – | – | – | – | – | 3.97±0.28 | 26.84±1.82 | 3.97±0.28 | 19.46±2.23 | ||
| T6 | – | – | – | – | – | – | – | 4.42±0.31 | 28.22±1.42 | 4.42±0.31 | 20.59±1.32 | ||
| T7 | – | – | – | – | – | – | – | 4.90±0.39 | 29.80±1.69 | 4.90±0.39 | 21.15±1.16 | ||
| T8 | – | – | – | – | – | – | – | 5.43±0.29 | 31.06±1.58 | 5.43±0.29 | 21.84±1.32 | ||
CBT, cortical bone trajectory; CT, computed tomography; SL, screw length; SD, screw diameter; LA, lateral angle; CA, cephalad angle.
Matsukawa et al. (45) investigated penetrating S1 endplate CBT (PECBT) technique, which angulating cranially in the sagittal plane penetrating the middle of the sacral endplate, and directed straight forward in the transverse plane. The mean cephalad angle was 30.7°±5.1°, and the mean length of trajectory was 31.5±3.5 mm. Additionally, in vitro biomechanical study showed that PECBT demonstrated an average of 141% higher insertional torque than the traditional monocortical technique.
Case presentation
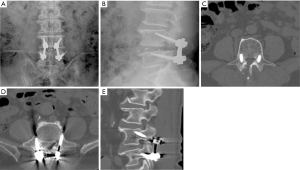
A 68-year-old male had low back pain radiating to left lower extremity, accompanying with intermittent claudication that lasted for 6 years and aggravated 2 weeks ago. This patient had failed to respond to conservative treatment which included physiotherapy and medication. Imaging studies showed central canal stenosis involving L4/5. The patient underwent L4/5 transforaminal lumbar interbody fusion with CBT screw fixation (Screw diameter: 5.5 mm, length: 35 mm). He had significant improvement in his back pain and neurogenic claudication postoperatively. Postoperative plain radiographs and CT scans show good CBT screws through the pedicles (Figure 4).
Indications and contraindications
The indications for CBT screw fixation include: (I) patient with osteopenia or osteoporosis who would obtained a more rigid structure from CBT screws which maximize thread contact with higher density cortical bone; (II) patients with diabetes or obesity who would benefit from a more medial starting point and less muscle dissection of CBT insertion; (III) CBT use as a rescue option in the setting of a failed or compromised pedicle screw construct in the lumbar spine; (IV) patients with symptomatic adjacent segment lumbar disease who was previously instrumented pedicles without removal of the pre-existing hardware; (V) a double trajectory technique, using both CBT technique and TPS, in the same pedicle, for instrumentation in a patient with severe osteoporosis.
The absolute contraindications for CBT screw fixation include a congenital pars defect, lack of cortical bone at the pars secondary to a wide decompression, and iatrogenic pars fracture. The relative contraindications include a narrow pars, congenital small pedicles, and severe spinal deformity with axial vertebral rotation.
Limitations of CBT screw fixation
The limitations for CBT screw fixation include: (I) lateralized trajectory and starting point around the pars, which may contribute to the development of pars fracture leading to fixation failure (46); (II) with the original CBT technique, starting point at inappropriate angles could cause nerve root disorders because the insertion points are positioned just above the nerve root; (III) the isthmus-guided CBT technique, screws are shorter and their insertion points closer to the cranial side than the original CBT technique, there is less bone cortex in contact with the screws, further biomechanical study should be carried out; (IV) without the usage of navigation system, isthmus-guided CBT technique need multiplanar fluoroscopy, which increases the risk of radiation exposure; (V) the starting point is medial on the pars, the surgeon sometimes needs to remove the inferior 1/2 of the spinous process to achieve the appropriate angulation for the trajectory; (VI) as a novel technique, CBT screw is not familiar to surgeon, so that it is important to make the strategy preoperatively (e.g., initial point, screw size, screw angle, and decompression width).
Conclusions and key points
- The CBT screw fixation is an anatomic feasible technique for lumbar, thoracic and sacral spine fixation, with potential advantages: less soft tissue dissection, and less risk of damage to nerve roots and vascular structures injuries;
- The CBT screw can be inserted percutaneously or using a free hand technique;
- The diameter of lumbar CBT screws ranges from 4.5 to 5.5 mm, and the length ranges from 25 to 35 mm;
- Several biomechanical studies demonstrated that CBT technique has equivalent or superior biomechanical properties of TPS;
- Retrospective, short term clinical outcomes reported in the literature show that CBT has lower blood loss than TPS;
- Further randomized controlled trials are needed to compare CBT vs. TPS techniques;
- CT image/Isthmus-guided CBT technique may provide a feasible and safe option for accurate screw placement.
Acknowledgements
Funding: This work was funded by the National Natural Science Foundation of China (81501933, 81572214), Zhejiang Provincial Natural Science Foundation of China (LY14H060008), Zhejiang Provincial Medical and Health Technology Foundation of China (2018KY129), Wenzhou leading talent innovative project (RX2016004) and Wenzhou Municipal Science and Technology Bureau (Y20170389). The funders had no role in the design, execution, or writing of the study.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.12.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Umeta RS, Avanzi O. Techniques of lumbar-sacral spine fusion in spondylosis: systematic literature review and meta-analysis of randomized clinical trials. Spine J 2011;11:668-76. [Crossref] [PubMed]
- Suk SI, Kim JH, Kim SS, et al. Pedicle screw instrumentation in adolescent idiopathic scoliosis (AIS). Eur Spine J 2012;21:13-22. [Crossref] [PubMed]
- Wu AM, Zou F, Cao Y, et al. Lumbar spinal stenosis: an update on the epidemiology, diagnosis and treatment. AME Med J 2017;2:63. [Crossref]
- Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [Crossref] [PubMed]
- Katz JN, Harris MB. Clinical practice. Lumbar spinal stenosis. N Engl J Med 2008;358:818-25. [Crossref] [PubMed]
- Ni WF, Huang YX, Chi YL, et al. Percutaneous pedicle screw fixation for neurologic intact thoracolumbar burst fractures. J Spinal Disord Tech 2010;23:530-7. [Crossref] [PubMed]
- Wu AM, Chen CH, Shen ZH, et al. The Outcomes of Minimally Invasive versus Open Posterior Approach Spinal Fusion in Treatment of Lumbar Spondylolisthesis: The Current Evidence from Prospective Comparative Studies. Biomed Res Int 2017;2017:8423638
- Tsuang FY, Chen CH, Kuo YJ, et al. Percutaneous pedicle screw placement under single dimensional fluoroscopy with a designed pedicle finder-a technical note and case series. Spine J 2017;17:1373-80. [Crossref] [PubMed]
- Song T, Hsu WK, Ye T. Lumbar pedicle cortical bone trajectory screw. Chinese Medical Journal 2014;127:3808-13. [PubMed]
- Wang Q, Huang M, Ou D, et al. One-stage extreme lateral interbody fusion and percutaneous pedicle screw fixation in lumbar spine tuberculosis. J Musculoskelet Neuronal Interact 2017;17:450-5. [PubMed]
- Davne SH, Myers DL. Complications of lumbar spinal fusion with transpedicular instrumentation. Spine 1992;17:S184-9. [Crossref] [PubMed]
- Halvorson TL, Kelley LA, Thomas KA, et al. Effects of bone mineral density on pedicle screw fixation. Spine 1994;19:2415-20. [Crossref] [PubMed]
- Okuyama K, Sato K, Abe E, et al. Stability of transpedicle screwing for the osteoporotic spine. An in vitro study of the mechanical stability. Spine 1993;18:2240-5. [Crossref] [PubMed]
- Weiser L, Huber G, Sellenschloh K, et al. Insufficient stability of pedicle screws in osteoporotic vertebrae: biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur Spine J 2017;26:2891-7. [Crossref] [PubMed]
- Hoppe S, Keel MJ. Pedicle screw augmentation in osteoporotic spine: indications, limitations and technical aspects. Eur J Trauma Emerg Surg 2017;43:3-8. [Crossref] [PubMed]
- Shea TM, Laun J, Gonzalez-Blohm SA, et al. Designs and techniques that improve the pullout strength of pedicle screws in osteoporotic vertebrae: current status. Biomed Res Int 2014;2014:748393
- Zhuang XM, Fu CF, Liu WG, et al. Biomechanical effect of the correction on the anchoring strength of de-orbiting S1 bicortical pedicle screw - An in-vitro investigation in normal and osteoporotic conditions. Clin Biomech (Bristol, Avon) 2016;36:26-31. [Crossref] [PubMed]
- Santoni BG, Hynes RA, McGilvray KC, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J 2009;9:366-73. [Crossref] [PubMed]
- Barakat AS, Owais T, Alhashash M, et al. Presentation and management of symptomatic central bone cement embolization. Eur Spine J 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Martínfernández M, Lópezherrradón A, Piñera AR, et al. Potential risks of using cement-augmented screws for spinal fusion in patients with low bone quality. Spine J 2017;17:1192-9. [Crossref] [PubMed]
- Phan K, Hogan J, Maharaj M, et al. Cortical Bone Trajectory for Lumbar Pedicle Screw Placement: A Review of Published Reports. Orthop Surg 2015;7:213-21. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Nemoto O, et al. Morphometric measurement of cortical bone trajectory for lumbar pedicle screw insertion using computed tomography. J Spinal Disord Tech 2013;26:E248-53. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Kato T, et al. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976) 2014;39:E240-5. [Crossref] [PubMed]
- Calvert GC, Lawrence BD, Abtahi AM, et al. Cortical screws used to rescue failed lumbar pedicle screw construct: a biomechanical analysis. J Neurosurg Spine 2015;22:166-72. [Crossref] [PubMed]
- Chen W, Wang H, Jiang J, et al. Anatomic study on lumbar cortical bone trajectory of adults Chinese J Orthop 2015;35:1213-20.
- Iwatsuki K, Yoshimine T, Ohnishi Y, et al. Isthmus‐guided Cortical Bone Trajectory for Pedicle Screw Insertion. Orthop Surg 2014;6:244-8. [Crossref] [PubMed]
- Senoglu M, Karadag A, Kinali B, et al. Cortical Bone Trajectory Screw for Lumbar Fixation: A Quantitative Anatomical and Morphometric Evaluation. Laboratory Investigation. World Neurosurg 2017;103:694-701. [Crossref] [PubMed]
- Ueno M, Sakai R, Tanaka K, et al. Should we use cortical bone screws for cortical bone trajectory? J Neurosurg Spine 2015;22:416-21. [Crossref] [PubMed]
- Baluch DA, Patel AA, Lullo B, et al. Effect of physiological loads on cortical and traditional pedicle screw fixation. Spine 2014;39:E1297-302. [Crossref] [PubMed]
- Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine 2013;38:635-41. [Crossref] [PubMed]
- Ueno M, Imura T, Inoue G, et al. Posterior corrective fusion using a double-trajectory technique (cortical bone trajectory combined with traditional trajectory) for degenerative lumbar scoliosis with osteoporosis: technical note. J Neurosurg Spine 2013;19:600-7. [Crossref] [PubMed]
- Rodriguez A, Neal MT, Liu A, et al. Novel placement of cortical bone trajectory screws in previously instrumented pedicles for adjacent-segment lumbar disease using CT image-guided navigation. Neurosurg Focus 2014;36:E9 [Crossref] [PubMed]
- Mizuno M, Kuraishi K, Umeda Y, et al. Midline lumbar fusion with cortical bone trajectory screw. Neurol Med Chir (Tokyo) 2014;54:716-21. [Crossref] [PubMed]
- Takata Y, Matsuura T, Higashino K, et al. Hybrid technique of cortical bone trajectory and pedicle screwing for minimally invasive spine reconstruction surgery: a technical note. J Med Invest 2014;61:388-92. [Crossref] [PubMed]
- Gonchar I, Kotani Y, Matsumoto Y. Cortical Bone Trajectory versus Percutaneous Pedicle Screw in Minimally Invasive Posterior Lumbar Fusion. Spine J 2014;14:S114-S5. [Crossref]
- Lee GW, Son JH, Ahn MW, et al. The comparison of pedicle screw and cortical screw in posterior lumbar interbody fusion: a prospective randomized noninferiority trial. Spine J 2015;15:1519-26. [Crossref] [PubMed]
- Orita S, Inage K, Kubota G, et al. One-Year Prospective Evaluation of the Technique of Percutaneous Cortical Bone Trajectory Spondylodesis in Comparison with Percutaneous Pedicle Screw Fixation: A Preliminary Report with Technical Note. J Neurol Surg A Cent Eur Neurosurg 2016;77:531-7. [Crossref] [PubMed]
- Glennie RA, Dea N, Kwon BK, et al. Early clinical results with cortically based pedicle screw trajectory for fusion of the degenerative lumbar spine. J Clin Neurosci 2015;22:972-5. [Crossref] [PubMed]
- Pacione D, Kim I, Wilson TA, et al. Cortical screw trajectory for instrumentation and fusion in the setting of osteopathic compression fracture allows for percutaneous kyphoplasty for adjacent level compression fractures. J Clin Neurosci 2015;22:899-904. [Crossref] [PubMed]
- Bogduk N, Long DM. The anatomy of the so-called “articular nerves” and their relationship to facet denervation in the treatment of low-back pain. J Neurosurg 1979;51:172-7. [Crossref] [PubMed]
- Matsukawa K, Yato Y, Hynes RA, et al. Cortical Bone Trajectory for Thoracic Pedicle Screws: A Technical Note. Clin Spine Surg 2017;30:E497-E504. [PubMed]
- Xuan J, Zhang D, Jin HM, et al. Minimally invasive cortical bone trajectory screws placement via pedicle or pedicle rib unit in the lower thoracic spine: a cadaveric and radiographic study. Eur Spine J 2016;25:4199-207. [Crossref] [PubMed]
- Chen D, Ni WF, Lin Y, et al. The Feasibility of cortical bone trajectory screw fixation for lower thoracic spine. AME Med J 2017;2:123. [Crossref]
- Sheng SR, Chen JX, Wei C, et al. Cortical bone trajectory screws for the middle-upper thorax: An anatomico-radiological study. Medicine 2016;95:e4676 [Crossref] [PubMed]
- Matsukawa K, Yato Y, Kato T, et al. Cortical bone trajectory for lumbosacral fixation: penetrating S-1 endplate screw technique: technical note. J Neurosurg Spine 2014;21:203-9. [Crossref] [PubMed]
- Mobbs RJ. The “Medio-Latero-Superior Trajectory Technique”: an Alternative Cortical Trajectory for Pedicle Fixation. Orthop Surg 2013;5:56-9. [Crossref] [PubMed]
Cite this article as: Feng ZH, Li XB, Tian NF, Sheng SR, Li YM, Phan K, Lin ZK, Joaquim AF, Xuan J, Lin Y, Wang XY, Matsukawa K, Zhang K, Zhao J, Ni WF, Wu AM. The technique of cortical bone trajectory screw fixation in spine surgery: a comprehensive literature review. AME Med J 2018;3:8.

