The impact of obesity through fat depots and adipokines on bone homeostasis
Introduction
Over the past 4 decades, obesity has become a major threat to public health worldwide (1). The Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group examined 9.1 million adults between 1980 and 2008 and reported that the global prevalence of obesity nearly doubled with approximately 1.5 billion adults having body mass index (BMI) of 25 or more (34%) and 500 million adults characterized as obese in 2008 (2). In a more recent analysis, the Global Burden of Disease Study 2013 revealed that the proportion of adults with BMI 25 or more, increased from 29% to 37% in men, and from 30% to 38% in women during 1980 to 2013 (3).
Obesity and osteoporosis are two of the most common chronic disorders with high prevalence rates, associated with increased morbidity and mortality (4). Research on the relationship between BMI/obesity and bone metabolism often yields contradictory results. Earlier studies showed that low body weight and recent weight loss were risk factors for osteoporosis and fractures, implying a positive correlation between body weight (or BMI) and bone mass (5,6). In agreement with this observation, obesity was commonly thought to be advantageous for maintaining healthy bones and high bone mineral density was observed in overweight individuals attributed to increased skeletal loading (7). In contrast, recent studies showed high risk of fracture and osteoporosis in people with high BMI (8,9). Patients with obesity had higher risk of fracture in humerus, ankle, and upper arm, compared to subjects with normal weight (7). Such statement was confirmed in the Osteoporotic Fractures in Men (MrOS) study, as obesity was shown to be a major risk factor for osteoporotic fractures (10). As well, in the Global Longitudinal study of Osteoporosis in Women (GLOW), obesity was associated with increased risk of ankle and upper leg fractures in postmenopausal women (11). In patients with type 2 diabetes mellitus (T2DM) characterized by obesity plus insulin resistance, the observed normal or high bone mass was accompanied by a greater risk of fractures (12).
Such paradox may be explained in part by the interplay between obesity and impaired bone metabolism, which is highly influenced by abnormal local fat deposition and secretions. As a consequence, the pattern of regional fat deposition into the subcutaneous and visceral compartments represents a stronger predictor of disease risk than overall fat mass (13,14). Recently, a new pathophysiological mechanism as emerged describing how the increased fat within the bone marrow, affects osteoblast differentiation and function, increases osteoclastic activity and disturbs mineralization (15). Furthermore, as adipose tissue is a highly dynamic organ with crucial endocrine and metabolic roles, cytokines (usually referred as adipokines) including leptin (16), adiponectin (17), omentin (18), etc., such production by adipose tissue is highly suspected to influence homeostasis between bone and fat.
Here, we review the interaction between adipose tissue and bone health by assessing the different deposition of adipose tissue and the influence of adipokines in bone metabolism.
Role of different deposition of adipose tissue interacting with bone homeostasis
The adipose function varies according to the fat deposition location and typically two main locations for adipose tissue are considered: subcutaneous and visceral deposition. Compared with subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT) is more cellular, vascular, innervated and employs more inflammatory and immune cells, lesser pre-adipocyte differentiating capacity and higher ratio of large adipocytes (19). Recently, the role of marrow adipose tissue (MAT) in bone metabolism was discussed (20). An inverse relationship between MAT and bone mineral density has been observed in several studies (21-23).
Subcutaneous vs. VAT
Obesity was initially assumed as a protection factor against fracture while clinical evidence was not consistent. Travison et al. (24) enrolled 1,171 men aged 30–79 years in a cross-sectional study, and BMD, bone material in cross-sections, bending strength, and propensity to buckle under compression were tested using dual energy X-ray absorptiometry (DXA). Fat mass and BMI had a negative association with hip strength after monitoring lean mass. The protective effect of BMI in preventing fracture was attributed to increased muscle mass accompanying elevated BMI rather than to adipose tissue. Similar conclusions were drawn with sexually mature adolescents and young adults aged 13 to 21 years (25). Following studies further suggested that fat mass was negatively related with bone mass in African and Chinese women (26,27). Zhang et al. (28) enrolled 347 Chinese obese females and 339 Chinese obese males and found that increased central body fat had an inverse association with total and leg BMD in females but not in males.
The complex relation between bone and adipose tissue was further explored by the different deposition of fat mass between SAT and VAT (29). The cross-sectional dimensions, and polar and principal moments of the femur, were obtained by computed tomography (CT) in 100 healthy women from USA, aged 15–25 years (29). Results showed that subcutaneous fat had a positive predictive value with femoral cross-sectional area, cortical bone area, principal moment maximum, principal moment minimum, and polar moment, but a negative effect was observed between visceral fat and all femoral bone phenotypes measured. Thus, although subcutaneous fat was beneficial to bone structure, visceral fat functions as a pathogenic fat depot. This was confirmed in a large cross-sectional study of 8,833 patients aged 18–64.9 years who underwent thoracic or abdominal CT scans; VAT areas, SAT areas, trabecular bone densities, and cortical bone densities at vertebral levels T7 to L5, were measured (30). Statistical significant correlations between VAT and decreased densities of cortical and trabecular bones were reported even after adjustment for age, sex, and BMI. Similarly, Campos et al. (31) performed a study including 125 postpubertal obese adolescents (45 boys and 80 girls) and found a negative relationship between BMD and insulin resistance, visceral fat, leptin concentration and visceral/subcutaneous fat ratio. A positive association between BMD and subcutaneous fat was found only in boys. To go further, the effect of fat distribution (visceral/subcutaneous) on bone quality and microarchitecture was investigated in 1,474 postmenopausal Korean women (32). The researchers reported that trabecular bone score (TBS) was not associated with total fat mass but it was negatively associated with trunk fat mass. Moreover, it was positively related with leg and gynoid fat mass and negatively related with android fat mass. Using linear regression models fed with age, BMI, and physical activity, they concluded that android fat, in contrast to gynoid fat, was inversely associated with TBS. In 228 healthy Chinese men aged from 38 to 89 years, android fat (and not gynoid, trunk, or limbs fat) showed significant inverse association with TBS. Furthermore, visceral fat was described as pathogenic fat harmful to TBS (33). Cohen et al. (34) enrolled 40 healthy premenopausal women to investigate the effects of obesity on bone resorption or formation. Bone density and trunk fat was measured by DXA while bone microarchitecture, stiffness, remodeling, and marrow fat were assessed in labeled transiliac bone biopsies. They concluded that premenopausal women with trunk adiposity had inferior bone quality, lower trabecular bone volume fraction and stiffness and noticeably lower bone formation.
MAT—a new view and target
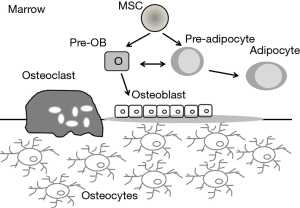
Recently, MAT has drawn much attention from researchers assessing bone homeostasis. Bone marrow is the only place where bone and fat lie adjacent to each other (21). Additionally, a mesenchymal progenitor can be found in the marrow that is able to generate osteoblasts, adipocytes, and myocytes (35). There is widespread support for the hypothesis that bone marrow mesenchymal stromal cells (BMSCs) enter only one lineage in a mutually exclusive manner and that this ‘choice’ is determined by an orderly fashion during maturation, which was controlled by specific transcription factors and hormones (36). The increase of MAT in metabolic disorders of low bone mass and the observation that osteoblasts and adipocytes derive from a common pool of mesenchymal progenitors, implies a balanced tradeoff between bone and fat mass, with the increased formation of adipocytes occurring at the expense of osteoblasts leading to lower bone mass (Figure 1) (4).
In patients with type 1 diabetes, high MAT was observed, which was accompanied with abnormal cortical bone geometry and high risk of fracture (37,38). In two mice models of spontaneous and induced type 1 diabetes, the proximal tibia exhibited marked increase in both MAT number and levels of adipocyte mRNA markers such as peroxisome proliferator-activated receptor gamma (PPARG) and fatty acid binding protein (FABP) 4 mRNA, along with decrease of mineral density and bone volume fraction mostly in trabecular and to a lesser extend in cortical bone, as well as in osteocalcin mRNA (39). PPARG is a ligand-dependent transcription factor that regulates genes involved in lipid and glucose homeostasis, including FABP4. In turn, FABP4 binds fatty acids and the FABP4/fatty acid complex activates PPARG in the nucleus under a positive feedback loop. Treatment with a PPARG antagonist, bisphenol A diglycidyl ether (BADGE), prevented accumulation of marrow adiposity but not bone loss in diabetes (40) while BADGE treatment in wildtype adult mice resulted in increased bone formation and decreased marrow adipogenesis (41).
In patients with type 2 diabetes, normal or high BMD together with elevated risk of fracture was observed; in contrast, high MAT was not detected (42,43). Recently, the altered composition of MAT in patients with type 2 diabetes was linked with bone fragility and diabetes (44). Sixty-nine postmenopausal women (36 subjects with spinal and/or peripheral fragility fractures and 33 non-fracture controls) were screened by magnetic resonance spectroscopy of the lumbar spine (LS); the BMD was determined using DXA of the hip and LS and quantitative computed tomography (QCT) of the LS. Diabetic individuals with fractures featured the lowest marrow unsaturation but the highest saturation. No association of marrow fat content with diabetes or fracture was observed. Yu et al. (45) studied 21 adults with morbid obesity, 8 of whom had T2DM and found that subjects with T2DM had higher volumetric BMD (vBMD) of the femoral neck and higher total MAT at the LS and femoral metaphysis compared to non-diabetic controls. Lipid unsaturation index (evaluated as olefinic protons to total lipid content ratio) was significantly lower at the femoral diaphysis in T2DM. LS vBMD was inversely correlated with LS MAT. An inverse association between SAT and diaphyseal MAT was observed while no associations were found for VAT (45).
In patients with osteoporosis, increased MAT was reported for the first time by Meunier et al. (46). A recent cross-sectional study of the Age Gene/Environment Susceptibility-Reykjavik cohort found that high MAT was negatively correlated to trabecular BMD in women, and positively correlated to common vertebral fractures in men (47). Both osteoporosis and osteopenia were associated with low proportion of unsaturated lipids, as assessed by proton magnetic resonance spectroscopy (1H-MRS) (48). In rodent models, aging was associated with significant marrow adiposity, and this finding has been linked to low trabecular bone mineral density in rodents (49) as well as in humans (47), suggesting a mutually exclusive process.
Adipokine—relationship between adipose tissue and bone homeostasis
Adipose tissue represents a dynamic and complex organ with endocrine, metabolic and immune regulatory roles. As a secretory organ, the characteristics of adipose tissue depend on the fat depots (visceral, subcutaneous or marrow) and the cellular composition (mature adipocytes, stromal-vascular cells, and nonfat immune cells including macrophages) (50). Hypertrophic adipocytes were related to dysregulated adipokine and chemokine production (51). In obese individuals, adipokines have been implicated in the pathogenesis of inflammation and insulin resistance. Thus, leptin, resistin, chemerin, and visfatin-1, were overexpressed, while adipokines with anti-inflammatory properties, such as adiponectin and omentin, were decreased (52,53).
Roles of leptin in bone metabolism
Leptin is one of the most important cytokines secreted from adipose tissue and its role is widely mentioned in energy homeostasis and regulation of energy expenditure. In recent years, we have realized that leptin also plays a major role in neuroendocrine regulation and bone metabolism (54,55). Leptin production has shown a positive correlation with BMI and fat mass (56). In mice with double null mutations in leptin gene (ob/ob mice) and leptin receptors (db/db), high bone mass phenotype was observed compared to wild type mice (57,58). Also, central administration of leptin gene therapy was able to correct skeletal abnormalities in ob/ob mice (59) suggesting that hypothalamic leptin has a definite role in normalizing bone growth. Recent studies showed that leptin has different effects on different parts of the skeleton. In ob/ob mice, enhanced bone formation was observed in vertebral length, lumbar bone mineral density as well as increased trabecular bone volume (60). However, bone formation of appendicular region was decreased, with shorter femora, lower BMD, reduced cortical thickness and trabecular bone volume when compared to wild type mice (61).
Leptin has an impact on bone homeostasis via central and peripheral pathway (Figure 2) (54). In the central nervous system, leptin regulates bone formation via the sympathetic nervous system (SNS) and ventromedial hypothalamus (VMH) (62) targeting osteoblasts but not osteoclasts. When leptin binds to its receptor on VMH neurons (Ob-Rb), signals are transmitted to osteoblasts via two sympathetic signal pathways (63): one of them suppresses osteoblast proliferation through downregulation of c-myc and increased expression of cyclin D and the other one promotes the resorption effects of osteoclasts through increased expression of the receptor activator of NF-B ligand (RANKL) through protein kinase A (PKA)-activating transcription factor (ATF)4 pathway (64). The 2-adrenergic receptor (Adrb2) is the vital receptor receiving the sympathetic signals from VHM. Two distinct downstream pathways transduce the signal: First, Adrb2 uses the transcription factor cAMP-response element binding protein (CREB) to regulate gene expression (65). Through CREB, sympathetic signaling activates the molecular clock and Ap-1 genes. The former inhibits the expression of the transcription factor c-myc and thereby downregulating the expression of Cyclin D1, which, in the end, leads to the suppression of osteoblasts proliferation (66). Second,
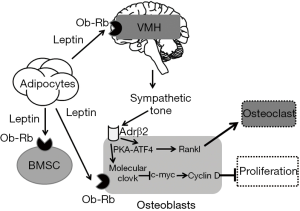
Peripheral leptin is synthesized and released into circulation by fat tissue. Leptin influences BMSCs, osteoblasts, osteoclasts, and chondrocytes (68). High affinity leptin receptors are present in human BMSCs (69,70). Leptin is able to enhance BMSC proliferation and differentiation of these progenitor cells into the osteoblastic lineage inhibiting their differentiation into adipocytes (71). Also, leptin affects autophagy in BMSCs, which protects BMSCs from apoptosis through AMPK and mTOR pathway (72,73). Leptin promotes osteoblasts proliferation, de novo collagen synthesis and in vitro mineralization, as well as cell survival and transition into preosteocytes (74). Leptin may also assist the osteoblastic signaling to osteoclasts (74). At the same time, leptin can inhibit osteoclast generation in vitro by increasing the expression of osteoprotegerin and decreasing the receptor activator of RANKL in stromal cells (75). Burguera et al. (76) reported that peripheral leptin administration had a protective effect on ovariectomy-induced bone loss in rats by increasing osteoprotegerin mRNA in osteoblasts. Martin et al. (77) presented that peripheral administration of leptin could prevent tissue-induced bone loss in rats through inhibition of bone resorption and delayed prevention of bone formation decreasing rate. Hamrick et al. (78) found that leptin administered peripherally could also lead to adipocyte apoptosis and increased bone formation in transgenic ob/ob mice.
Roles of adiponectin in bone metabolism
Circulating adiponectin levels are negatively correlated with decreased BMI in obese subjects (79). Adiponectin prevents inflammation, oxidation and fibrosis in adipose tissue through the inhibition of the NF-kB pathway (80), which controls the expression of tumor necrosis factor- (TNF-), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and endothelial-leukocyte adhesion molecule 1 (ELAM-1) expression (50). In addition, adiponectin primes human monocyte into anti-inflammatory M2 macrophages as well as from “harmful” Th1/17 to “beneficial” Th2/Treg by inhibiting TLR4-mediated NF-kB activation (81).
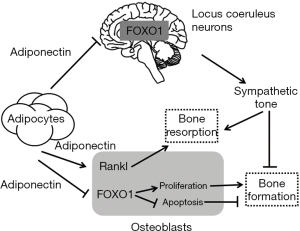
On bone mass accrual, adiponectin exerts its influence through two opposite mechanisms, one local on osteoblasts and one central on the SNS (Figure 3) (82). In osteoblasts, it directly increases RANKL expression and decreases forkhead box protein FoxO1 activity in a PI3 kinase-dependent manner. However, these effects are rapidly masked due to the more powerful and central role of adiponectin. Indeed, adiponectin exerts its effect in the brain on the sympathetic tone, inhibiting the activity of the SNS, followed by increasing bone formation and bone mass, decreasing energy expenditure and blood pressure (82). As a consequence, adiponectin indirectly inhibits RANKL expression and increases FoxO1 activity, which results in osteoblast proliferation and increased bone formation, bone mass, and circulating osteocalcin levels (82). This is why young knockdown adiponectin−/− mice exhibited high bone mass and bone formation factors (83). Remarkably, the two opposite modes of action of adiponectin target the same molecules but differences are related to the effect on Cyclin D1. Indeed, in osteoblasts, adiponectin directly inhibits osteoblast proliferation and Cyclin D1 accumulation while through its central signaling adiponectin indirectly favors osteoblast proliferation and Cyclin D1 accumulation.
Other adipokines involved in bone metabolism
Resistin is mainly produced by articular white adipose tissue and participates in adipogenesis, insulin resistance and inflammatory processes. Fasting plasma levels of resistin were significantly correlated with femur BMD in patients with osteoporosis (84). The plasma visfatin levels were positively correlated to BMD L2–L4 in men with metabolic syndromes (85). High concentrations of visfatin underexpress factors like SOX9 and type II collagen which are considered essential for the maintenance of chondrocyte phenotype (86). Omentin-1 induces human osteoblast proliferation via the PI3K/Akt signaling pathway (87). Omentin-1 in synovial fluid might be proved as a potential biomarker for the degenerative process and symptomatic severity of knee osteoarthritis (88).
Conclusions
The complex relationship between fat and bone can be differentiated into systemic and local interaction (89). The systemic interaction between fat and bone refers to adipokines released by peripheral adipose tissue (such as subcutaneous and visceral) and affect bone metabolism either in a negative or positive manner (90,91). In contrast, the local interaction refers to the fat interaction with bone cells within the bone marrow (20,92).
The role of adipokines in regulating bone metabolism is not completely clear. Leptin, one of the most important adipokines for bone, regulates bone homeostasis via central signaling while it can directly regulate osteoblastogenesis and marrow adipogenesis via peripheral action. On the other hand, adiponectin, has the inverse effect of leptin on bone metabolism but also functions through central and peripheral pathways. However, the role of some adipokines (such as resistin, visfatin and omentin-1) in bone metabolism has not been fully investigated yet. The interactions among different adipokines are also poorly understood.
Moreover, the role of local adipose tissue remains a challenging question for many reasons: first, the classification of MAT and its function is not clear since the negative association of high marrow adiposity and low bone mass is convoluted and varies with age. Second, the relationship between MAT and white adipose depots (including SAT and VAT) is highly complex because bone marrow adipocytes tend to accumulate when white fat depots are depleted. Nevertheless, the precise effects of SAT/VAT vs. MAT on bone metabolism are still under investigation. Third, the origin of MAT and how these cells interact with osteoblasts and hematopoietic elements is largely unknown.
In conclusion, although obesity and different depots of fat clearly affect bone homeostasis through multiple detailed pathways, the exact involved mechanisms still need further investigation.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.12.08). The authors have no conflicts of interest declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab 2015;66:7-12. [Crossref] [PubMed]
- Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557-67. [Crossref] [PubMed]
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [Crossref] [PubMed]
- Devlin MJ, Rosen CJ. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol 2015;3:141-7. [Crossref] [PubMed]
- Nguyen ND, Pongchaiyakul C, Center JR, et al. Abdominal fat and hip fracture risk in the elderly: the Dubbo Osteoporosis Epidemiology Study. BMC Musculoskelet Disord 2005;6:11. [Crossref] [PubMed]
- Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab 2006;91:2534-41. [Crossref] [PubMed]
- Caffarelli C, Alessi C, Nuti R, et al. Divergent effects of obesity on fragility fractures. Clin Interv Aging 2014;9:1629-36. [PubMed]
- Palermo A, Tuccinardi D, Defeudis G, et al. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int J Environ Res Public Health 2016;13:E544 [Crossref] [PubMed]
- Gonnelli S, Caffarelli C, Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab 2014;11:9-14. [PubMed]
- Nielson CM, Marshall LM, Adams AL, et al. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS). J Bone Miner Res 2011;26:496-502. [Crossref] [PubMed]
- Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med 2011;124:1043-50. [Crossref] [PubMed]
- Leslie WD, Rubin MR, Schwartz AV, et al. Type 2 diabetes and bone. J Bone Miner Res 2012;27:2231-7. [Crossref] [PubMed]
- Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372-9. [Crossref] [PubMed]
- Mori Y, Hoshino K, Yokota K, et al. Differences in the pathology of the metabolic syndrome with or without visceral fat accumulation: a study in pre-diabetic Japanese middle-aged men. Endocrine 2006;29:149-53. [Crossref] [PubMed]
- Gunaratnam K, Vidal C, Gimble JM, et al. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology 2014;155:108-16. [Crossref] [PubMed]
- Barbour KE, Zmuda JM, Boudreau R, et al. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporos Int 2012;23:1699-710. [Crossref] [PubMed]
- Barbour KE, Zmuda JM, Boudreau R, et al. Adipokines and the risk of fracture in older adults. J Bone Miner Res 2011;26:1568-76. [Crossref] [PubMed]
- Xie H, Xie PL, Wu XP, et al. Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc Res 2011;92:296-306. [Crossref] [PubMed]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev 2010;11:11-8. [Crossref] [PubMed]
- Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci 2014;1311:14-30. [Crossref] [PubMed]
- Mendonca ML, Batista SL, Nogueira-Barbosa MH, et al. Primary Hyperparathyroidism: The Influence of Bone Marrow Adipose Tissue on Bone Loss and of Osteocalcin on Insulin Resistance. Clinics (Sao Paulo) 2016;71:464-9. [Crossref] [PubMed]
- Casazza K, Hanks LJ, Hidalgo B, et al. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone 2012;50:23-7. [Crossref] [PubMed]
- Di Iorgi N, Rosol M, Mittelman SD, et al. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab 2008;93:2281-6. [Crossref] [PubMed]
- Travison TG, Araujo AB, Esche GR, et al. Lean mass and not fat mass is associated with male proximal femur strength. J Bone Miner Res 2008;23:189-98. [Crossref] [PubMed]
- Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab 2007;92:143-7. [Crossref] [PubMed]
- Sotunde OF, Kruger HS, Wright HH, et al. Lean Mass Appears to Be More Strongly Associated with Bone Health than Fat Mass in Urban Black South African Women. J Nutr Health Aging 2015;19:628-36. [Crossref] [PubMed]
- Shao HD, Li GW, Liu Y, et al. Contributions of fat mass and fat distribution to hip bone strength in healthy postmenopausal Chinese women. J Bone Miner Metab 2015;33:507-15. [Crossref] [PubMed]
- Zhang J, Jin Y, Xu S, et al. Associations of fat mass and fat distribution with bone mineral density in Chinese obese population. J Clin Densitom 2015;18:44-9. [Crossref] [PubMed]
- Gilsanz V, Chalfant J, Mo AO, et al. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 2009;94:3387-93. [Crossref] [PubMed]
- Zhang P, Peterson M, Su GL, et al. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr 2015;101:337-43. [Crossref] [PubMed]
- Campos RM, Lazaretti-Castro M, Mello MT, et al. Influence of visceral and subcutaneous fat in bone mineral density of obese adolescents. Arq Bras Endocrinol Metabol 2012;56:12-8. [Crossref] [PubMed]
- Kim JH, Choi HJ, Ku EJ, et al. Regional body fat depots differently affect bone microarchitecture in postmenopausal Korean women. Osteoporos Int 2016;27:1161-8. [Crossref] [PubMed]
- Lv S, Zhang A, Di W, et al. Assessment of Fat distribution and Bone quality with Trabecular Bone Score (TBS) in Healthy Chinese Men. Sci Rep 2016;6:24935. [Crossref] [PubMed]
- Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab 2013;98:2562-72. [Crossref] [PubMed]
- Bianco P, Robey PG, Saggio I, et al. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther 2010;21:1057-66. [Crossref] [PubMed]
- Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci 2009;66:236-53. [Crossref] [PubMed]
- McCabe LR. Understanding the pathology and mechanisms of type I diabetic bone loss. J Cell Biochem 2007;102:1343-57. [Crossref] [PubMed]
- McCabe L, Zhang J, Raehtz S. Understanding the skeletal pathology of type 1 and 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr 2011;21:187-206. [Crossref] [PubMed]
- Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 2007;148:198-205. [Crossref] [PubMed]
- Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. J Cell Physiol 2006;209:967-76. [Crossref] [PubMed]
- Duque G, Li W, Vidal C, et al. Pharmacological inhibition of PPARgamma increases osteoblastogenesis and bone mass in male C57BL/6 mice. J Bone Miner Res 2013;28:639-48. [Crossref] [PubMed]
- Baum T, Yap SP, Karampinos DC, et al. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging 2012;35:117-24. [Crossref] [PubMed]
- Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011;305:2184-92. [Crossref] [PubMed]
- Patsch JM, Li X, Baum T, et al. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res 2013;28:1721-8. [Crossref] [PubMed]
- Yu EW, Greenblatt L, Eajazi A, et al. Marrow adipose tissue composition in adults with morbid obesity. Bone 2017;97:38-42. [Crossref] [PubMed]
- Meunier P, Aaron J, Edouard C, et al. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 1971;147-54. [Crossref] [PubMed]
- Schwartz AV, Sigurdsson S, Hue TF, et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab 2013;98:2294-300. [Crossref] [PubMed]
- Yeung DK, Griffith JF, Antonio GE, et al. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging 2005;22:279-85. [Crossref] [PubMed]
- Duque G, Rivas D, Li W, et al. Age-related bone loss in the LOU/c rat model of healthy ageing. Exp Gerontol 2009;44:183-9. [Crossref] [PubMed]
- Azamar-Llamas D, Hernandez-Molina G, Ramos-Avalos B, et al. Adipokine Contribution to the Pathogenesis of Osteoarthritis. Mediators Inflamm 2017;2017:5468023.
- Maury E, Ehala-Aleksejev K, Guiot Y, et al. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab 2007;293:E656-65. [Crossref] [PubMed]
- Cheon DY, Kang JG, Lee SJ, et al. Serum Chemerin Levels are Associated with Visceral Adiposity, Independent of Waist Circumference, in Newly Diagnosed Type 2 Diabetic Subjects. Yonsei Med J 2017;58:319-25. [Crossref] [PubMed]
- Kang DR, Yadav D, Koh SB, et al. Impact of Serum Leptin to Adiponectin Ratio on Regression of Metabolic Syndrome in High-Risk Individuals: The ARIRANG Study. Yonsei Med J 2017;58:339-46. [Crossref] [PubMed]
- Chen XX, Yang T. Roles of leptin in bone metabolism and bone diseases. J Bone Miner Metab 2015;33:474-85. [Crossref] [PubMed]
- Upadhyay J, Farr OM, Mantzoros CS. The role of leptin in regulating bone metabolism. Metabolism 2015;64:105-13. [Crossref] [PubMed]
- Neumann E, Junker S, Schett G, et al. Adipokines in bone disease. Nat Rev Rheumatol 2016;12:296-302. [Crossref] [PubMed]
- Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 2000;100:197-207. [Crossref] [PubMed]
- Turner RT, Kalra SP, Wong CP, et al. Peripheral leptin regulates bone formation. J Bone Miner Res 2013;28:22-34. [Crossref] [PubMed]
- Iwaniec UT, Boghossian S, Lapke PD, et al. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides 2007;28:1012-9. [Crossref] [PubMed]
- Hamrick MW, Pennington C, Newton D, et al. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004;34:376-83. [Crossref] [PubMed]
- Parm AL, Jurimae J, Saar M, et al. Plasma adipocytokine and ghrelin levels in relation to bone mineral density in prepubertal rhythmic gymnasts. J Bone Miner Metab 2011;29:717-24. [Crossref] [PubMed]
- Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell 2002;111:305-17. [Crossref] [PubMed]
- Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab 2006;4:341-8. [Crossref] [PubMed]
- Schwetz V, Pieber T, Obermayer-Pietsch B. The endocrine role of the skeleton: background and clinical evidence. Eur J Endocrinol 2012;166:959-67. [Crossref] [PubMed]
- Benovic JL, Bouvier M, Caron MG, et al. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol 1988;4:405-28. [Crossref] [PubMed]
- Fu L, Patel MS, Bradley A, et al. The molecular clock mediates leptin-regulated bone formation. Cell 2005;122:803-15. [Crossref] [PubMed]
- Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet 2003;4:638-49. [Crossref] [PubMed]
- Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 2011;301:E567-84. [Crossref] [PubMed]
- Pedroso JA, Buonfiglio DC, Cardinali LI, et al. Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab 2014;3:608-18. [Crossref] [PubMed]
- Hess R, Pino AM, Rios S, et al. High affinity leptin receptors are present in human mesenchymal stem cells (MSCs) derived from control and osteoporotic donors. J Cell Biochem 2005;94:50-7. [Crossref] [PubMed]
- Astudillo P, Rios S, Pastenes L, et al. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem 2008;103:1054-65. [Crossref] [PubMed]
- Wang L, Hu X, Zhu W, et al. Increased leptin by hypoxic-preconditioning promotes autophagy of mesenchymal stem cells and protects them from apoptosis. Sci China Life Sci 2014;57:171-80. [Crossref] [PubMed]
- Singh R, Xiang Y, Wang Y, et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 2009;119:3329-39. [PubMed]
- Gordeladze JO, Drevon CA, Syversen U, et al. Leptin stimulates human osteoblastic cell proliferation, de novo collagen synthesis, and mineralization: Impact on differentiation markers, apoptosis, and osteoclastic signaling. J Cell Biochem 2002;85:825-36. [Crossref] [PubMed]
- Holloway WR, Collier FM, Aitken CJ, et al. Leptin inhibits osteoclast generation. J Bone Miner Res 2002;17:200-9. [Crossref] [PubMed]
- Burguera B, Hofbauer LC, Thomas T, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology 2001;142:3546-53. [Crossref] [PubMed]
- Martin A, de Vittoris R, David V, et al. Leptin modulates both resorption and formation while preventing disuse-induced bone loss in tail-suspended female rats. Endocrinology 2005;146:3652-9. [Crossref] [PubMed]
- Hamrick MW, Della-Fera MA, Choi YH, et al. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 2005;20:994-1001. [Crossref] [PubMed]
- Nigro E, Scudiero O, Monaco ML, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int 2014;2014:658913.
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000;102:1296-301. [Crossref] [PubMed]
- Ohashi K, Parker JL, Ouchi N, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010;285:6153-60. [Crossref] [PubMed]
- Kajimura D, Lee HW, Riley KJ, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab 2013;17:901-15. [Crossref] [PubMed]
- Wu Y, Tu Q, Valverde P, et al. Central adiponectin administration reveals new regulatory mechanisms of bone metabolism in mice. Am J Physiol Endocrinol Metab 2014;306:E1418-30. [Crossref] [PubMed]
- Mohiti-Ardekani J, Soleymani-Salehabadi H, Owlia MB, et al. Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone metabolic markers in osteoporosis patients. J Bone Miner Metab 2014;32:400-4. [Crossref] [PubMed]
- Iacobellis G, Iorio M, Napoli N, et al. Relation of adiponectin, visfatin and bone mineral density in patients with metabolic syndrome. J Endocrinol Invest 2011;34:e12-5. [Crossref] [PubMed]
- Yang Z, Huang CY, Candiotti KA, et al. Sox-9 facilitates differentiation of adipose tissue-derived stem cells into a chondrocyte-like phenotype in vitro. J Orthop Res 2011;29:1291-7. [Crossref] [PubMed]
- Wu SS, Liang QH, Liu Y, et al. Omentin-1 Stimulates Human Osteoblast Proliferation through PI3K/Akt Signal Pathway. Int J Endocrinol 2013;2013:368970.
- Xu L, Zhu GB, Wang L, et al. Synovial fluid omentin-1 levels are inversely correlated with radiographic severity of knee osteoarthritis. J Investig Med 2012;60:583-6. [Crossref] [PubMed]
- Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol 2008;20:429-34. [Crossref] [PubMed]
- Bermeo S, Gunaratnam K, Duque G. Fat and bone interactions. Curr Osteoporos Rep 2014;12:235-42. [Crossref] [PubMed]
- Scotece M, Conde J, Abella V, et al. Bone metabolism and adipokines: are there perspectives for bone diseases drug discovery? Expert Opin Drug Discov 2014;9:945-57. [Crossref] [PubMed]
- Fazeli PK, Horowitz MC, MacDougald OA, et al. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab 2013;98:935-45. [Crossref] [PubMed]
Cite this article as: Jin J, Wang Y, Jiang H, Kourkoumelis N, Renaudineau Y, Deng Z. The impact of obesity through fat depots and adipokines on bone homeostasis. AME Med J 2018;3:10.