
Endovascular treatment for the acute ischemic stroke: the past and the future
Endovascular treatment for the acute ischemic stroke (AIS): the past and the future
As one of the main causes of death and disability worldwide, AIS needs to be treated as quickly as possible in order for any causal treatment to be effective (1). Occlusion of large arteries, such as the terminal parts of the internal carotid artery (ICA) and the main stem of the middle cerebral artery (MCA), often result in poor outcomes of the AIS patients (2). Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) has evolved to the standard treatment of AIS within the 4.5 hours
Clear limitations of intravenous thrombolysis are the short therapeutic window (≤4.5 hours), haemorrhagic complications, and the overall recanalization rate. In clinical practice it is difficult to judge the thrombus volume, but easy to evaluate the site of occlusion. While MCA branch occlusions show a recanalization rate of approximately 60% six hours after stroke onset, MCA mainstem occlusions have a chance below 30% to fully recanalize within 6 hours (5). For increasing revascularization rates in large artery occlusions and to improve outcome other therapies such as endovascular treatment need to be explored.
The strategies of endovascular treatment
Intra-arterial (IA) thrombolysis
For AIS patients with large artery occlusion, IA thrombolysis can be used to improve the efficiency of drug delivery into the thrombus. With the aim of recanalizing the occlusion, the microcatheter is placed at the proximal end of the occlusion and a high dose of fibrinolytic agent is released in close proximity of the thrombus (6). IA thrombolysis requires comparably smaller drug dosages to achieve recanalization than i.v. thrombolysis and the technical of intervention is considered simple. Although the technique is regarded technically simple it is more time-consuming than mechanical thrombectomy. In very proximal occlusion, recanalization rates are reported modest (7). But in more distal occlusions, such as M2 branches, the recanalization rates are higher. After IA thrombolysis, brain hemorrhagic and distal emboli are the common complications (8).
Prototype study for this approach is the pro-urokinase in acute cerebral thromboembolism (PROACT) II trial (9). However, pro-urokinase never has been clinically available, so that the study has a proof-of-principle character.
IA thrombolysis has never been approved by the US Food and Drug Administration (FDA) but is still frequently used around the world for emergency treatment of patients with ICA, MCA or basilar artery occlusion. During the intervention, researchers find that IA thrombolysis often fails to improve the outcome of patients, because large thrombi usually required a long time to be dissolved (10).
Endovascular ultrasound-enhanced thrombolysis
The use of direct intravascular ultrasound for clot lysis was first described in 1974 in an animal model (11). The energies for endovascular ultrasound vary between 0.2–2.0 W/cm2, and the frequencies between 20 KHz to 2 MHz. Ultrasound can promote microstreaming, which is the motion of fluid around the thrombus (12). Ultrasound generates pressure waves, which increase the permeation of rt-PA into the fibrin network. Ultrasound energy enhances the binding of rt-PA to the fibrin within a matrix, and weakens the fibrin cross-links at the same time (12). Ultrasound transducers also can be incorporated into the catheters for IA delivery of the thrombolytic drug. The IA ultrasound device can emit a power of 400 mW to enhance the effect of thrombolytic drugs, which has been tested in the phase II–III of interventional management of stroke (IMS) trials (13).
Endovascular angioplasty and stenting
Balloon angioplasty and stenting in cerebral artery occlusion is a technique that is similar to the approach taken in acute myocardial infarction. Cerebral blood vessels are more prone to dissection because they are without firm muscular support. In the cerebral vessels, the approach to the occlusion position is often tortuous making navigation more difficult. To remove the obstacles of endovascular angioplasty and stenting in AIS patients, many catheters and devices designed for the cerebral artery have been developed in recent years.
In a retrospective analysis of 19 patients, permanent stent afforded 79% successful recanalization. Due to the small sample, there were no cases of symptomatic intracranial hemorrhage (sICH) in the study (14). The results showed that stent-assisted recanalization is associated with a high recanalization rate. However, disadvantage of permanent stenting, such as the administration of dual antiplatelet therapy, is significant. The loading dose of clopidogrel can increase the risk of symptomatic ICH after the recanalization. At present, the use of permanent stents to the treatment of patients with cerebral artery occlusion has been restricted with the advent of embolectomy techniques.
Thrombectomy techniques
Compared with pharmacologic thrombolysis, thrombectomy techniques have many advantages, including higher rates of recanalization and low risk of ICH. Thrombectomy techniques can currently be classified into 4 groups depending on the device used: the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) Retriever, the Penumbra System, the Solitaire FR revascularization device and TREVO Pro retriever (15).
The MERCI devices received FDA approval in 2004, which are deployed into the thrombus and works as a cork screw like retrieval tool (16). To achieve flow arrest and prevent distal embolization, the balloon of the guiding catheter is inflated proximal to the clot. After the removal of the clot, the proximal balloon is deflated and the circulation is restored.
The penumbra stroke system was approved by the FDA in 2007, which uses an aspiration catheter along with a separator to debulk and remove the thrombus (17). Stent-based mechanical thrombectomy such as solitaire FR Revascularization Device is a new concept in AIS treatment. With the self-expanding stents partially deployed, clot enmeshed into the stent, and the vessel is gently aspirated. Based on results from the Solitaire with the Intention for Thrombectomy (SWIFT) trial, the solitaire stent has been extensively used in Europe since 2010, and received FDA approval in March 2012 (18) (Figure 1). As same type device, TREVO Pro Stent Retriever mechanical embolectomy device approved by the FDA in August 2012, based on the results of the TREVO 2 trial (19).
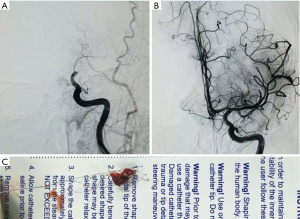
Important published studies about the endovascular treatment
Pro-urokinase in acute cerebral thromboembolism (PROACT) II
To confirm the safety and efficacy of IA thrombolysis in AIS patients caused by MCA occlusion, Prolyse in Acute Cerebral Thromboembolism (PROACT) II was designed (20). Patients in the group treated with IA pro-urokinase plus heparin within 3 to 6 hours from stroke onset get a higher 90 days good outcome rate compared with the group treated with heparin alone (40% vs. 25%). The rates of complete recanalization, defined as Thrombolysis in Myocardial Infarction (TIMI) grade 3, was 19% in the pro-urokinase plus heparin group, and only 4% in the heparin alone group. But the rate of sICH was 10% in the treated patients, and which was just 2% in the controls. The mortality rates in the two groups have no significant difference (25% vs. 27%) (9).
The IMS studies I and II
The IMS I and II were designed to examine the effects of an initially lower dose of IV rt-PA (0.6 mg/kg) followed by IA rt-PA or/and IA ultrasound when recanalization was not achieved (18). The rates of recanalization (TIMI 2 to 3) were 56% in IMS-I and 60% in IMS-II. About 46% of IMS-II patients reached a mRS score of 0 to 2 at 3 months after stroke. In both studies, the mortality at the 3 months was 16%, and sICH rates were 6.3% and 9.9% respectively (21).
In the IMS trials, the method of reduced-dose IV rt-PA followed by endovascular therapy was used as a bridging concept. A large proportion of patients undergoing thrombectomy had i.v. rt-PA (0.9 mg/kg) prior catheter according the results of ECASS III 3.0–4.5 h after onset of stroke (22). The previous observational studies also supported the full dose IV rt-PA used, because the risks of sICH are acceptable (23).
MERCI study and Multi-MERCI study
The MERCI study explored the safety and efficacy of the MERCI device in 151 patients. Patients enrolled in this study had both anterior and posterior circulation strokes. All the patients had large artery occlusions, and were treated within 8 hours of stroke onset (24). The results showed that the rates of recanalization to TIMI 2 to 3 in the MERCI study connected with a significant improvement was achieved in 46%. At 1 month after stroke, 22.6% of patients reached a mRS score ≤2, and 34.1% of patients a NIHSS score improvement ≥10 points. At 3 months after stroke, the rate of mRS ≤2 improved to 27.7%, and NIHSS improvement ≥10 points to 32.4% respectively. The mortality lay at 43.5% at 90 days, and sICH was observed in 11 of 141 (7.8%) (24).
In the Multi-MERCI study, IV or IA rt-PA, mechanical clot disruption, and other adjunctive therapies were allowed prior using the MECRI device. Some of the patients were treated with the newer generation L5 device (25). All the 160 patients were treated within 8 hours of stroke onset, and 29% of the participants received IV rt-PA without recanalization before the procedure. The results showed that vessel recanalization was achieved in 55% of patients with the retriever alone, and improved to 68% when adjunctive therapies were used. Compared with in ICA and M1-MCA occlusions, the recanalization rates were higher in the posterior circulation (88% and 80% respectively). At 1 month, mRS ≤2 was achieved in 36% and NIHSS ≥10 improved to 26% of participants. Nine point eight percent of the participant suffered sICH, and the mortality was 34% at 90 days. Results showed that treatment with IV rt-PA before MERCI device deployment did not increase the risk of sICH. Multivariate analysis showed that recanalization was related to good outcome. Older age and higher admission NIHSS score were associated with mortality and poorer functional outcome.
SWIFT trial
The participants of the SWIFT trial were all AIS patients. All patients received treatment with the Solitaire (n=58) or MERCI device (n=55) within 8 hours of symptom onset. Compared with the MERCI group, the recanalization (TIMI 2 to 3) rate in the Solitaire group was higher (83% vs. 48%), and the rate of favorable outcome (mRS ≤2) at 90 days was significantly increased. The mortality from any causes by 90 days in the Solitaire group was 9%, and which was 12% in the MERCI group (26).
Negative trials
IMS-III was a placebo controlled trail evaluating endovascular intervention as a rescue therapy in patients who failed to improve after a reduced-dose IV rt-PA (0.6 mg/kg) thrombolysis within 3 hours of stroke symptom onset. Many endovascular therapy methods including IA rt-PA, EKOS catheter, MERCI devices, and the Penumbra system were used in the study. However, stent retrievers were only rarely imployed. The planned study size was 900 participants but the trial was prematurely halted because of futility after 656 patients. The results showed that good functional outcome (mRS ≤2) at 90 days did not differ between the two groups. Rates of mortality and sICH were similar between the groups (27).
In the SYNTHESIS Expansion trail patients with AIS were included within 4.5 hours after symptom onset. Both the endovascular therapy group and the IV rt-PA group enrolled 181 patients each. The median time to initiation of therapy was 1 hour longer in the endovascular arm. Only 56 patients were treated with device, and only 18 patients were treated with a stent retriever. The results showed that good functional outcome (mRS ≤2) rates were not different between the groups in the intention-to-treat analysis after adjustment for age and stroke severity (42.0% vs. 46.4%). Adverse events of the two groups, including sICH, did not differ significantly (28).
Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR-RESCUE) was a trial assessing the value of endovascular therapy using the MERCI device or the Penumbra device and IA rt-PA when necessary within 8 hours of stroke symptom onset in relation to whether patients had ischemic penumbra as defined by MR diffusion-perfusion mismatch. On final review, 58% of the 118 patients had a favorable penumbral pattern. The results showed that although patients with penumbra had smaller final stroke volumes, the overall rates of favorable outcome were low even among the patients with penumbra on MRI. Recanalization rates with endovascular therapy group were 67% among the participants with favorable penumbral pattern and 77% in those without penumbra (29,30).
Delayed time to treatment, inclusion of patients without artery occlusion or with distal occlusion, less effective devices used are common causes of the lack of benefit from embolectomy in these trials
Recent trials showed benefit of thrombectomy
From 2015, five new trails showing the superiority of endovascular treatment were published. The trials had a similar design and inclusion criteria: the therapeutic window for the endovascular treatment was 6 hours, proximal intracranial occlusion in the anterior circulation were required.
EXTEND-IA is the smallest trial demonstrating benefit of thrombectomy over intravenous thrombolysis with inclusion of only 70 patients who were within 4.5 hours of symptom onset and had confirmed internal carotid or MCA occlusion (31). The participants were randomized to two groups: IV rt-PA only and additionally thrombectomy with the Solitaire FR stent retriever. The primary outcomes of the trail were reperfusion at 24 hours and a reduction of the NIHSS score >8 points within 3 days. Compared with the control group, the rates of reperfusion at 24 hours was higher in the endovascular treatment group (100% vs. 37%, P<0.001), and the rate of early neurological improvement was higher (80% vs. 37%, P=0.002). At 30 days outcome was better in the thrombectomy arm (mRS ≤2; 71% vs. 40%; P=0.001). The mortality and sICH rates did not differ at 90 days (32).
The multicenter randomized clinical trial of endovascular treatment in the Netherlands (MR CLEAN) was a large trial with 500 patients from 16 different centers. Patients with anterior circulation large artery occlusion were randomly allocated to endovascular treatment plus usual care or usual care only. The use of intravenous rt-PA was not required in the usual care group, but 89% of the total sample received intravenous rt-PA before randomization. The primary outcome of MR CLEAN was a measure of functional independence (mRS ≤2) 90 days after the intervention. The results showed an absolute difference of 13.5 percentage points [95% confidence interval (CI), 5.9 to 21.2] in the rate of functional independence (mRS ≥2) in favor of the intervention (32.6% vs. 19.1%).
ESCAPE was a trail with 316 patients from 22 centers from the USA, Canada, South Korea, Ireland and the UK. The patients were randomized into standard care or standard care plus endovascular treatment. Unlike the other trails, the participants’ treatment window was up to 12 hours after the stroke onset. The rate of functional independence at 1 month was significantly higher in the interventional group (53% vs. 29.3%, P<0.001). The mortality rate in the endovascular thrombectomy group was reduced, while the rates of sICH were not significantly different. However, the rate of new ischemic strokes in other vascular territories was higher in the intervention group (33).
Because of the significant efficacy, the SWIFT PRIME trail was terminated early in 2014. From 2012 to 2104, 196 participants from 39 centers were randomized to IV rt-PA alone or to additional endovascular treatment. The Solitaire FR or Solitaire 2 devices were used in the endovascular treatment group. The primary outcome was functional independence (mRS ≤2) at 3 months. The rate of functional independence was much higher in the endovascular treatment group compared with controls (60% vs. 35%, P<0.001). Mortality and sICH rates at 90 days were similar in the two groups (34).
The RESVASCAT trial was halted prematurely when positive results from other RCTs had been published. Two hundred and six eligible patients were enrolled. The participants include no recanalization after 30 minutes of the onset of IV rt-PA or ineligible IV rt-PA ineligible. The enrolled patients were randomized to endovascular treatment or medical therapy alone. Solitaire stent retriever was used in the study. Rates of functional independence (mRS ≤2) were higher in the endovascular treatment arm (43.7% vs. 28.3%). The safety variables did not differ between the two groups (35) (Tables 1,2).
Table 1
| Study name | Country | Recruitment onset and end | Device used | Sample size | Groups | Therapeutic window | Culprit artery | Primary outcome (90 days mRS) |
|---|---|---|---|---|---|---|---|---|
| Trials showed no benefit of thrombectomy | ||||||||
| IMS III | USA, Canada, Australia, Europe | 2006.8–2012.7 | MERCI retriever, Penumbra system or Solitaire FR or endovasc t-PA | 656 | Endovascular Thrombectomy + IV rt-PA vs. IV rtPA | rt-PA was given within 3 hrs of symptom onset; randomisation done 40 minutes after rt-PA infusion onset | ICA, M1 and BA | Proportion with a mRS ≤2: 40.8% and 38.7% (95% CI, −6.1 to 9.1) |
| MR RESCUE | USA | 2004.5–2011 | MERCI retriever, Penumbra’ system | 118 | Endovascular Thrombectomy vs. best medical care | ≤8 hrs from stroke onset | ICA, M1 and M2 | Mean score on the mRS: 3.9 vs. 3.9, P=0.99 |
| SYNTHESIS EXP | Italy | 2008.2–2012.4 | Not specified | 362 | Endovascular therapies vs. IV rtPA | ≤6 hrs from stroke onset | Not specified | Proportion with a mRS <2: 30.4% and 34.8% (95% CI, −14.1 to 5.2) |
| Trials showed benefit of thrombectomy | ||||||||
| EXTEND- IA | Australia and New Zealand | 2012.8–2014.10 | Solitare FR | 70 | Endovascular Thrombectomy + IV rt-PA vs. IV rt-PA |
≤6 hrs from stroke onset | ICA, M1, M2 | mRS 71% vs. 40% Generalized OR =2.0; 95% CI, 1.2–3.8 |
| MR CLEAN | Netherlands | 2010.12–2014.3 | Devices approved by the FDA or CE and approved by their steering committee | 500 | Endovascular therapies* vs. IV rt-PA | ≤6 hrs from stroke onset | ICA, M1, M2, A1 and A2 | Proportion with a mRS ≤2: 13.5% (95% CI, 5.9–21.2) |
| ESCAPE | Canada, USA, South Korea, Ireland, UK | 2013.2–2014.10 | Use of retrievable Stents were recommended |
316 | Endovascular Thrombectomy+ IV rt-PA vs. IV rtPA | ≤12 hrs from stroke onset | ICA, M1, M1-MCA (2 or more M2-MCAs) | Proportion with a mRS ≤2: 53% and 29.3%; RR, 1.4; (95% CI, 1.4–2.4); OR, 2.6; (95% CI, 1.7–3.8) |
| SWIFT PRIME | USA/Europe | 2012.11–2015.1 | Solitaire FR or Solitaire 2 | 196 | Endovascular Thrombectomy vs. IV rt-PA | ≤6 hrs from stroke onset | ICA, M1 | Proportion with a mRS ≤2: 60% and 35%; RR =1.70; (95% CI, 1.23–2.33) |
| REVASCAT | Spain | 2012.2–2014–12 | Solitaire | 206 | Endovascular Thrombectomy vs. best medical care** |
≤8 hrs from stroke onset | ICA, M1 | Proportion with a mRS ≤2: 43.7% and 28.2%; OR =2.1 (95% CI, 1.2–4.0) |
*, intra-arterial t-PA, mechanical thrombectomy or a combination of both; **, standard care (including IV rt-PA). IV r-tPA, intravenous recombinant tissue-type plasminogen activator; ICA, internal carotid artery; MCA, middle cerebral artery; M1, segment 1 middle cerebral artery; M2, segment 2 middle cerebral artery; A1, segment 1 anterior cerebral artery; A2, segment 2 anterior cerebral artery; RCTs, randomized controlled trials; AIS, acute ischemic stroke.
Table 2
| Study | Country | Mortality | sICH |
|---|---|---|---|
| IMS III | USA, Canada, Australia, Europe | At 90 days 19.1% vs. 21.6% (P=0.52) | After rt-PA initiation 6.2% vs. 5.9% (P=0.83) |
| MR RESCUE | USA | At 90 days 18.7% vs. 24.1% | At 90 days 5% vs. 3.7% (P=0.24) |
| SYNTHESIS EXP | Italy | At day 7, 8% vs. 6% (P=0.53) | At day 7 6% vs. 6% (P=0.99) |
| EXTEND-IA | Australia and New Zealand | At 90 days 9% vs. 20% (P=0.18) | 0% vs. 6% (P=0.49) |
| MR CLEAN | Netherlands | At 30 days 18.9% vs. 18.4% | At 90 days 7.7% vs. 6.4% |
| ESCAPE | Canada, USA, South Korea, Ireland, UK | At 30 days 10.4% vs. 19% | At 30 days 3.6% vs. 2.7% |
| SWIFT PRIME | USA/Europe | At 90 days 9% vs. 12% (P=0.50) | At 27 hrs 0% vs. 3% (P=0.12) |
| REVASCAT | Spain | At 90 days 18% vs. 15.5% (P=0.60) | At 90 days 1.9% vs. 1.9% (P=1.00) |
The five recently trials published from 2014 have shown a superiority of interventional treatment plus IV rt-PA over IV rt-PA alone. The therapeutic windows are wider in the recent trails: 3 of them within 6 hours of symptom onset, 1 within 8 hours, and 1 within 12 hours. Beside the therapeutic window, the devices used, patients inclusion criteria were all important reasons of the superiority of the endovascular treatment (36).
In the three initial trails, one of the main limitations was the fact that first generation devices were used. Recent trials suggest that the recently developed stent retriever i.e., Solitaire FR are more effective than the first generation devices. The recanalization rates were improved and the time to achieve recanalization was shortend with stent retrievers. The results of the recently published positive trials reflect the superiority of these new devices.
The inclusion criteria of the trails are believed to greatly influence the positive results. Cases with proven proximal artery occlusion are less prone to respond to IV-rtPA and are associated with more voluminous thrombi. Eligible patients had an occlusion of the ICA, MCA or anterior cerebral artery (ACA).
Recent systematic review and meta-analysis of recent trials yielded a risk ratio of 1.56 (95% CI, 1.38 to 1.75) for good functional outcomes and 0.86 (95% CI, 0.69 to 1.06) for mortality, without heterogeneity among the results of the studies. Moderate to high quality evidence suggests that for patients with anterior circulation ischaemic stroke, bridge treatment provides beneficial functional outcomes, without increased detrimental effects within 6 to 8 hours (37).
What next?
At present, some RCTs are still recruiting or planned. The details of these studies are summarized in Table 3. Most frequent comparison is between endovascular thrombectomy plus IV rt-PA with IV rt-PA alone.
Table 3
| Study | Country | Intervention groups | Device type brand | Sample size | Therapeutic window | Culprit artery | Recruitment onset/end | Current status |
|---|---|---|---|---|---|---|---|---|
| PISTE | United Kingdom | Endovasc. Thrombectomy + IV rt-PA vs. IV rt-PA | Not specified mechanical thrombectomy | 800 | ≤5.5 hrs | ICA, M1, M2 | 2012.12–2017.8 | This study is currently recruiting participants |
| BASICS | Italy, Netherlands, Switzerland | Endovasc. Thrombectomy + IV rt-PA vs. IV rt-PA | MERCI Trevo, Penumbra, Solitaire | 750 | ≤6 hrs | Basilar artery | 2011.10–2017.10 | This study is currently recruiting participants |
| POSITIVE | USA | Endovascular Thrombectomy vs. best medical care | Penumbra, Solitaire and TREVO | 750 | ≤12 hrs | ICA, M1 | 2013.9–2016.5 | This study is currently recruiting participants |
| DAWN | USA | Endovascular Thrombectomy vs. best medical care | Trevo | 500 | 6 to 24 hrs | ICA, M1 | 2014.6–2017.7 | This study is currently recruiting participants |
| RESILIENT | Brazil | Endovascular Thrombectomy vs. best medical care | Solitaire FR | 690 | ≤7.5 hrs | ICA, M1 | 2015.3–2018.3 | Not yet recruiting |
| WASSABI | USA | Endovascular thrombectomy vs. IV rt-PA vs. Standard medical care* | Merci Penumbra | 90 | Unknown time of onset but less than 24 hours since last seen normal | MCA | 2011.11–2014.2 | Status unknown |
| BEST | China | Endovascular treatment + standard medical therapy vs. standard medical* therapy alone | Solitaire FR, Trevo | 344 | ≤8 hrs | Basilar artery | 2015.4–2018.3 | This study is currently recruiting participants |
*, standard of care in acute ischemic stroke including intravenous thrombolysis. AIS, acute ischemic stroke; IV r-tPA, intravenous recombinant tissue-type plasminogen activator; ICA, internal carotid artery; MCA, middle cerebral artery; M1, segment 1 middle cerebral artery; M2, segment 2 middle cerebral artery; RCTs, randomized controlled trials.
At present, the sample size of the studies about the thrombectomy is relatively modest. In the real world, whether intravascular therapy can benefit more stroke patients remains a problem. In many areas, IV rtPA is the preferred method for treatment of acute stroke due to the lack of endovascular treatment techniques. With the continuous improvement of diagnostic neuroradiology services, more and more patients can benefit from the IV rtPA. In the future, the model can also be used for the implementation of endovascular therapy.
To maximise the benefit and minimise the harm and costs of treatment, it is important to identify the patients who are most likely to respond to endovascular treatment. To achieve this goal, clinicians have to make decisions about the suitability of endovascular thrombectomy using patients clinical and image information. Researchers developed multivariable prediction models that combine baseline risks and clinical and imaging features to estimate the effect of treatment. The models performed moderately well to estimate the benefit of endovascular treatment in individual patients. When the patients complete the imaging examination and begin the standard IV rt-PA treatment, the doctor and the family can use the calculator online to decide whether to further the endovascular treatment. This model also can help AIS patients and their families making decisions at a critical time. More studies are needed to incorporate findings from imaging studies into the model and compare the effect of these models with clinical results (38).
The penumbra is defined as reversible ischemia can be saved with successful reperfusion. With successful recanalization, the patients can be differentiated into reversible and viable ischemia up to 12 hours after onset according pretreatment perfusion imaging. However, most studies derived the imaging-based identification of penumbra from untreated patients. As such, advanced penumbral imaging was not considered with a major effect on treatment outcomes. Future penumbral imaging that derived from patients who have undergone prompt reperfusion may provide endovascular treatment opportunity that is not determined only by the time window (39).
At present, the favorable results for endovascular treatment were achieved at the background of IV t-PA. When no significant clinical response of IV rt-PA was observed, the patients were enrolled in the endovascular treatment group. But such nonresponse does not mean that the IV rt-PA complete futility, because IV t-PA could enhance subsequent endovascular treatment by platelet aggregation through the way of fibrinolysis and inhibition of shear stress. Even if IV rt-PA is not effective in all patients, such therapy may have supporting effects on endovascular treatment. Moreover, in the subgroups of the patients who received previous IV rt-PA and those who did not, recanalization degree and procedure duration might be different (40). Future trials of endovascular treatment might lead to a renewed appreciation of IV rt-PA or confirm the exactly effect of endovascular treatment in suitable patients.
Anesthesia used during endovascular treatment is also an important question in need to be answered. In recent trials, general anesthesia was used only in a small part of the participants. It is unknown whether the patients with general anesthesia had differences in outcome as compared with the patients with local anesthesia. A recent meta-analysis and systematic review found outcomes among AIS patients who had undergone general anesthesia was poorer than among those who had undergone conscious sedation and local anesthesia (41). The rate of respiratory complications was increased and the rate of good functional outcome was decreased in the general anesthesia group. The procedure time between the two groups did not differ. However, this question has not been definitely answered yet.
In recent trails, participants enrolled in the studies often had high stroke severity. Patients with severe stroke may be more likely to reach improved functional outcome. But in the real world, many patients with low stroke severity also need to be treated with appropriate methods. More characterization of the patients enrolled in future trials is needed to determine who will benefit from endovascular therapy.
Acknowledgements
Funding: None
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.12.11). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Natarajan SK, Karmon Y, Snyder KV, et al. Prospective acute ischemic stroke outcomes after endovascular therapy: a real-world experience. World Neurosurg 2010;74:455-64. [Crossref] [PubMed]
- Zhu W, Xiao L, Lin M, et al. Large-Vessel Occlusion Is Associated with Poor Outcome in Stroke Patients Aged 80 Years or Older Who Underwent Intravenous Thrombolysis. J Stroke Cerebrovasc Dis 2016;25:2712-6. [Crossref] [PubMed]
- Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870-947. [Crossref] [PubMed]
- de Los Ríos la Rosa F, Khoury J, Kissela BM, et al. Eligibility for Intravenous Recombinant Tissue-Type Plasminogen Activator Within a Population: The Effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke 2012;43:1591-5. [Crossref] [PubMed]
- Wunderlich MT, Goertler M, Postert T, et al. Recanalization after intravenous thrombolysis: does a recanalization time window exist? Neurology 2007;68:1364-8. [Crossref] [PubMed]
- Kan PT, Orion D, Yashar P, et al. Intra-arterial thrombolysis and thrombectomy for acute ischemic stroke: technique and results. J Neurosurg Sci 2011;55:151-60. [PubMed]
- Maas MB, Furie KL, Lev MH, et al. National Institutes of Health Stroke Scale score is poorly predictive of proximal occlusion in acute cerebral ischemia. Stroke 2009;40:2988-93. [Crossref] [PubMed]
- Ma QF, Chu CB, Song HQ. Intravenous versus intra-arterial thrombolysis in ischemic stroke: a systematic review and meta-analysis. PLoS One 2015;10:e0116120 [Crossref] [PubMed]
- Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology 2001;57:1603-10. [Crossref] [PubMed]
- Jeong HS, Song HJ, Kim SB, et al. A comparison of stent-assisted mechanical thrombectomy and conventional intra-arterial thrombolysis for acute cerebral infarction. J Clin Neurol 2013;9:91-6. [Crossref] [PubMed]
- Trübestein G, Engel C, Etzel F, et al. Thrombolysis by ultrasound. Clin Sci Mol Med Suppl 1976;3:697s-8s. [PubMed]
- Lauer CG, Burge R, Tang DB, et al. Effect of ultrasound on tissue-type plasminogen activator-induced thrombolysis. Circulation 1992;86:1257-64. [Crossref] [PubMed]
- Kramer C, Aguilar MI, Hoffman-Snyder C, et al. Safety and efficacy of ultrasound-enhanced thrombolysis in the treatment of acute middle cerebral artery infarction: a critically appraised topic. Neurologist 2011;17:346-51. [Crossref] [PubMed]
- Levy EI, Ecker RD, Horowitz MB, et al. Stent-assisted intracranial recanalization for acute stroke: early results. Neurosurgery 2006;58:458-63; discussion 458-63. [Crossref] [PubMed]
- Baker WL, Colby JA, Tongbram V, et al. Neurothrombectomy devices for the treatment of acute ischemic stroke: state of the evidence. Ann Intern Med 2011;154:243-52. [Crossref] [PubMed]
- Akins PT, Amar AP, Pakbaz RS, et al. Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol 2014;35:524-8. [Crossref] [PubMed]
- Yoo AJ, Frei D, Tateshima S, et al. The Penumbra Stroke System: a technical review. J Neurointerv Surg 2012;4:199-205. [Crossref] [PubMed]
- SWIFT trial of delayed elective intervention v conservative treatment after thrombolysis with anistreplase in acute myocardial infarction. SWIFT (Should We Intervene Following Thrombolysis?) Trial Study Group. BMJ 1991;302:555-60. [Crossref] [PubMed]
- Flint AC, Xiang B, Gupta R, et al. THRIVE score predicts outcomes with a third-generation endovascular stroke treatment device in the TREVO-2 trial. Stroke 2013;44:3370-5. [Crossref] [PubMed]
- Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 1999;282:2003-11. [Crossref] [PubMed]
- Gruber A. Interventional management of stroke. Stroke 2008;39:1663-4. [Crossref] [PubMed]
- Bluhmki E, Chamorro A, Dávalos A, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol 2009;8:1095-102. [Crossref] [PubMed]
- IMS Study Investigators. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904-11. [Crossref] [PubMed]
- Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432-8. [Crossref] [PubMed]
- Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205-12. [Crossref] [PubMed]
- Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241-9. [Crossref] [PubMed]
- Hirsch JA, Gonzalez RG. Understanding IMS III: old data shed new light on a futile trial. J Neurointerv Surg 2014;6:3-4. [Crossref] [PubMed]
- Kidwell CS, Jahan R, Saver JL. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013;368:2434-5. [Crossref] [PubMed]
- Kidwell CS, Jahan R, Alger JR, et al. Design and rationale of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) Trial. Int J Stroke 2014;9:110-6. [Crossref] [PubMed]
- Parsons MW, Albers GW MR. RESCUE: is the glass half-full or half-empty? Stroke 2013;44:2055-7. [Crossref] [PubMed]
- Campbell BC, Mitchell PJ, Yan B, et al. A multicenter, randomized, controlled study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits with Intra-Arterial therapy (EXTEND-IA). Int J Stroke 2014;9:126-32. [Crossref] [PubMed]
- McDowell MM, Ducruet AF. Time Is Brain: A Critical Analysis of the EXTEND-IA and ESCAPE Trials. World Neurosurg 2015;83:949-51. [Crossref] [PubMed]
- Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019-30. [Crossref] [PubMed]
- Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285-95. [Crossref] [PubMed]
- Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296-306. [Crossref] [PubMed]
- Muir KW, White P. HERMES: messenger for stroke interventional treatment. Lancet 2016;387:1695-7. [Crossref] [PubMed]
- Rodrigues FB, Neves JB, Caldeira D, et al. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ 2016;353:i1754. [Crossref] [PubMed]
- Hunter RM, Davie C, Rudd A, et al. Impact on clinical and cost outcomes of a centralized approach to acute stroke care in London: a comparative effectiveness before and after model. PLoS One 2013;8:e70420 [Crossref] [PubMed]
- Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013;368:914-23. [Crossref] [PubMed]
- Moftakhar P, English JD, Cooke DL, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke 2013;44:243-5. [Crossref] [PubMed]
- Brinjikji W, Murad MH, Rabinstein AA, et al. Conscious sedation versus general anesthesia during endovascular acute ischemic stroke treatment: a systematic review and meta-analysis. AJNR Am J Neuroradiol 2015;36:525-9. [Crossref] [PubMed]
Cite this article as: Liu R, Li W, Stolz E. Endovascular treatment for the acute ischemic stroke: the past and the future. AME Med J 2018;3:15.

