Association between CYP17A1 polymorphisms and response to abiraterone in patients with metastatic castration-resistant prostate cancer: a systematic review
Introduction
Prostate cancer (PC) is expected to affect 161,360 men in the US, accounting for almost 20% of new cancer cases diagnosed in 2017 (1,2). Due to the availability of PSA, most patients are diagnosed with PC in an early stage. At the same time, metastatic disease is only found in 4% of new cases in America and nearly 10% to 20% in Europe (3,4).
Despite apparently effective primary treatment is in general performed, more than 30% of PC patients experience disease progression. Many of these patients and almost all metastatic receive androgen deprivation therapy (ADT) with or without chemotherapy. However, almost all patients eventually experience disease progression to the castration-resistant state. The median time of this is approximately 1 year (5,6). 5-year survival rate of patients with metastatic disease is limited to 25–30% (7).
Abiraterone is an oral, selective and irreversible inhibitor of the cytochrome P450 17-hydrolase (CYP17A1) enzyme (8). One of the functions of abiraterone is to suppress the biosynthesis of testosterone in adrenal glands, testicles and tumour microenvironment, accounting for CYP17A1 catalyzing key reactions in sex-steroid hormone biosynthesis (9). Two phase III trials demonstrated a clear improvement in overall survival (OS) and progression-free survival (PFS) in patients with CRPC (10,11). Based on these data, abiraterone was approved in the United States and Europe for the treatment of CPRP patients regardless of docetaxel treatment (3). However, response to abiraterone is highly heterogeneous with many patients experiencing early failure of therapy. So, it is of great importance to identify particular patients who may benefit the most from the treatment of abiraterone, so that patients who are unlikely to benefit can be allocated to one of other therapies for management of CRPC. A biomarker that could help in personalized selection is likely to improve survival while reducing adverse events in this late stage of PC.
CYP17A1 is located on chromosome 19q23.4 and is clarified to be correlated to cardiovascular diseases through affecting blood pressure (12). CYP17A1 is an important target in treatment of PC. A PC experimental model indicated that alterations of CYP14A1 activity may be mechanisms of resistance to hormonal treatment (13,14). In one retrospective study, CYP17A1 polymorphisms appeared to be associated with the median PFS and OS in patients with CPRC (15). Conflicting results on CYP17A1 polymorphisms on abiraterone response have been reported (16,17).
Therefore, we conducted this systematic review to provide a more comprehensive understanding.
Material and methods
Search strategy
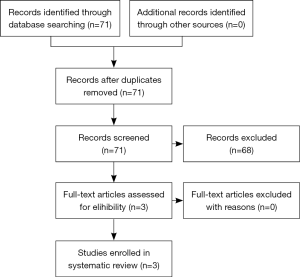
Potential articles included up to date, June 7, 2017. We conducted a systematic search on search engines such as PubMed, EMBASE and Web of Science, based on the ‘PRISMA’ guideline (18). There was no languages limitation. We used the following search term strategies: (“steroid 17-alpha-hydroxylase”, or “CYP17A1”), (“abiraterone”) and (“metastatic castration-resistant prostate cancer”, or “mCRPC”). To discover all studies, we screened the references of all relevant publications. A flow diagram of the research selection process is shown in Figure 1.
Inclusion criteria and exclusion criteria
The inclusion criteria were shown as follows: (I) studies assessing the relationship between CYP17A1 polymorphisms and outcomes of mCRPC patients treated with abiraterone; and (II) different studies without overlapped available data. The following exclusion criteria were applied: (I) studies missing outcomes of patients with mCRPC; and (II) overlapped data appearing in studies.
Data extraction
All valuable data of included studies were extracted by two independent investigators (X Z and X L). The following information was extracted: name of first author, year of publication, ethnicity, numbers of patients, age, PSA, abiraterone treatment scheme, sample collection method, CYP17A1 polymorphisms, Gleason score, Eastern Cooperative Oncology Group (ECOG) performance status, site of metastasis and number of previous chemotherapeutic lines. Hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CI) in PFS and OS were else extracted from included studies. All detailed information mentioned above is shown in Tables 1-4. In addition, Newcastle-Ottawa Scale (NOS) was adopted to evaluate the quality of all studies based on three domains: selection of cohort, comparability of cohorts, assessment of outcomes. The NOS ranges from 0 to 9. The ≥7 was identified to distinguish studies with high quality.
Table 1
| First author | Year | Ethnicity | No. of patients | Age# | PSA* | Treatment | Detected sample | CYP17A1 polymorphism | Gleason score | ECOG performance status | Sites of metastasis | No. of previous chemotherapeutic lines | NOS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6–7 | 8–9 | Unknown | 0–1 | 2 | Bone metastases | Soft tissue metastases | 1 | 2 or more | |||||||||||||
| Samanta Salvi (17) | 2016 | Caucasian | 64 | 73 [57–90] | 33.1a (0.35–1,501) | Abiraterone/prednisone | Blood | rs743572/rs10883783/rs17115100/rs284849 | 23 | 36 | 5 | 57 | 1 | 55 | 47 | 30 | 34 | 9 | |||
| Moritz Binder (16) | 2016 | NR | 87 | 73 [54–90] | 66b (0–100) | Abiraterone/prednisone | Blood | rs743572/rs4919685/rs17115100/rs2486758 | NR | NR | NR | NR | NR | 73 | 43 | 56 | NR | 7 | |||
| Samanta Salvi (15) | 2015 | NR | N:38 | N:73 [57–87] | N:21.9a (1–1,501) | Abiraterone/prednisone | Blood | Normal | N:16 | N:22 | N:- | N:36 | N:2 | N:31 | N:8 | N:20 | N:8 | 9 | |||
| A:15 | A:76 [57–86] | A:294.2a (13.8–1,435) | Amplified | A:7 | A:7 | A:1 | A:10 | A:5 | A:13 | A:4 | A:5 | A:10 | |||||||||
#, median age at start of abiraterone treatment (years, range); *, median baseline PSA level; a, ng/mL (range); b, ng/dL (range).NR, not reported; N, normal; A, amplified; ECOG, Eastern Cooperative Oncology Group.
Table 2
| First author | CYP17A1 polymorphism | Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Samanta Salvi | Amplified | 2.20 | 1.02–4.77 | 0.0450 | 0.92 | 0.37–2.29 | 0.8576 | |
| Normal | ||||||||
CYP17A1, cytochrome P450 17α-hydrolase; mCRPC, metastatic castration-resistant prostate cancer; HR, hazard ration; CI, confidence interval.
Table 3
| First author | CYP17A1 polymorphism | Biochemical response to treatment | Time to biochemical progression | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | HR | 95% CI | P value | |||
| Moritz Binder | rs17115100 | 0.82 | 0.31–2.19 | 0.689 | 0.95 | 0.67–1.35 | 0.693 | |
| rs743572 | 1.13 | 0.58–2.20 | 0.718 | 1.03 | 0.73–1.46 | 0.889 | ||
| rs4919685 | 1.24 | 0.64–2.40 | 0.524 | 0.82 | 0.47–1.42 | 0.429 | ||
| rs2486758 | 0.22 | 0.07–0.63 | 0.005 | 2.23 | 1.39–3.56 | <0.001 | ||
CYP17A1, cytochrome P450 17α-hydrolase; mCRPC, metastatic castration-resistant prostate cancer; OR, odds ratio; HR, hazard ration; CI, confidence interval.
Table 4
| First author | CYP17A1 polymorphism | Allele/genotype | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median months | 95% CI | P value | Median months | 95% CI | P value | ||||
| Samanta Salvi | rs743572 | AA | 7.0 | 4.1–12.7 | 0.6474 | 11.8 | 8.8–19.0 | 0.4825 | |
| GA + GG | 7.2 | 4.2–11.0 | 17.7 | 10.8–26.4 | |||||
| rs17115100 | GG | 6.8 | 4.6–10.5 | 0.4054 | 14.9 | 10.4–22.3 | 0.9825 | ||
| TG + TT | 9.5 | 2.4–15.0 | 17.7 | 6.6–26.4 | |||||
| rs10883783 | TT | 8.5 | 6.4–12.7 | 0.5586 | 17.7 | 9.6–22.0 | 0.7668 | ||
| TA + AA | 5.4 | 3.4–11.0 | 17.6 | 10.5–NR | |||||
| rs284849 | GG | 9.2 | 4.0–11.4 | 0.8282 | 18.6 | 11.4–NR | 0.5188 | ||
| TG + GG | 6.7 | 4.3–9.2 | 11.8 | 9.2–22.3 | |||||
CYP17A1, cytochrome P450 17α-hydrolase; mCRPC, metastatic castration-resistant prostate cancer; PFS, progression-free survival; OS, overall survival; CI, confidence interval.
Results
A total of 3 separate studies with 204 individual patients were found (15-17). The median age at start of abiraterone treatment ranged from 54 to 90 years old, and DNA was extracted from blood in all patients. Prior to the start of abiraterone treatment, median baseline PSA level, ECOG performance status, site of metastasis and number of previous chemotherapeutic lines containing docetaxel were assessed in two studies to identify whether patients met eligibility criteria. The third study only reported sites of metastasis, median baseline PSA level and previous ADT use as well as exposure to docetaxel, enzalutamide and cabazitaxel. Ethnicity of patients was mentioned in only one study. The detailed information of these studies is shown in Table 1.
Summary of results of the association between CYP17A1 polymorphisms and outcomes of mCRPC patients treated with abiraterone are shown in Tables 2-4. All patients received abiraterone acetate and prednisone, 28-day cycles of 1,000 mg abiraterone acetate daily with 5 mg prednisone twice-daily. Median PFS and OS of patients were reported in two studies. Biochemical response to treatment was defined as PSA decrease of ≥50% after starting abiraterone acetate and prednisone. Time to biochemical progression was set as PSA increase of >25% on two consecutive tests at least two weeks apart. Salvi et al. reported CYP17A1 copy number variations had an association with worse prognosis of abiraterone therapy in mCRPC patients. According to their retrospective analysis, patients with amplified CYP17A1 had significantly a shorter median PFS compared to those with normal CYP17A1 (HR =2.20; 95% CI: 1.02–4.77; P=0.045). This study revealed no statistic association between CYP17A1 copy number variations and OS (HR =0.92; 95% CI: 0.37–2.29; P=0.86) (Table 2) (15). Furthermore, Binder et al. reported that the rs2486758 polymorphism (allele C vs. T) was significantly associated with a poor biochemical response (OR =0.22; 95% CI: 0.07–0.63; P=0.005) and a short time to biochemical progression (HR =2.23; 95% CI: 1.39–3.56; P<0.001) (Table 3) (16). However, no significant associations between single-nucleotide polymorphisms (SNPs) in CYP17A1 and clinical outcome, such as PFS and OS, were reported by Salvi et al. (Table 4) (17).
Discussion
Abiraterone acetate irreversibly inhibits CYP17. CYP17 is a critical enzyme in the testosterone biosynthesis, playing an essential role in two sequential reactions: (I) transforming pregnenolone and progesterone to their 17-hydroxy derivatives; and (II) taking part in the formation of dehydroepiandrosterone (DHEA) and androstenedione. Besides the aforementioned information, one study indicated anti-tumor role of abiraterone by activating D4A metabolite, presumably through the blockade of multiple steroidogenic enzymes and antagonism of the androgen receptor (19). Treatment with abiraterone and prednisone has been demonstrated to greatly improve OS in patients with mCRPC regardless of docetaxel pretreatment (20-25). Recently, a network meta-analysis suggested that abiraterone is the second-most efficacious drug for improving OS in mCRPC patients, after Enzalutamide (26). Though grade 1 or 2 treatment-related adverse events are observed, abiraterone plus prednisone is generally well tolerated in well selected patients (11,27). In addition, a meta-analysis revealed Grade 3–4 complications (fatigue, back pain, anaemia, and bone pain) appeared in less than 10% of patients compared to placebo (28), and only heart failure and grade 3 hyper-transaminase required interruption of abiraterone (29).
Identification of variations in the particular predictive targets of outcome in mCRPC patients treated with abiraterone could assist in distinguishing who will actually benefit from this therapy. However, neither Gleason Score nor type and duration of prior endocrine therapies were evaluated as predictive elements (30,31). CYP17A1 is a potential biomarker that can be future assessed. It is the target of abiraterone and its polymorphisms might predict response to abiraterone in mCRPC patients.
In our review of literature, we could demonstrate that CYP17A1 polymorphisms was statistically associated with the outcomes of mCRPC patients treated with abiraterone. Patients with amplified CYP17A1 seem to experience a worse PFS (15), and amplification of CYP17A1 was associated with a higher expression of CYP17A1. When abiraterone was taken by men, CYP17A1 molecules were partly restricted and cancer was capable of going on with biosynthesis of testosterone (30,32). Modification of splicing process and mechanisms, as well as other pathways, may be utilized by SNPs in gene to change its expression level (33,34). The rs2486758 polymorphism was a predictor of patients with mCRPC (16). The rs2486758 is located in the promoter region of CYP17A1 and consequent up-transcription of CYP17A1 may be responsible for the increased risk (35). However 4 common SNPs, rs743572, rs17115100, rs10883783 and rs284849, were genotyped, but none should to be significantly associated with clinical outcomes in mCRPC patients. The drug’s wide therapeutic window may be responsible for this result. To reduce the false positive rate, large case series studies and investigations of other SNPs based on better comprehension of therapeutic window of abiraterone are still needed (17).
Our review suffers from several limitations. First, only 3 eligible studies focusing on CYP17A1 polymorphism and outcome of mCRPC patients treated with abiraterone were included in this systematic review. More high-quality, larger sample size studies are required to acquire further confirmation. Second, CYP17A1 polymorphisms were correlated with outcome in only two studies, which limits our conclusion.
In conclusion, we found that CYP17A1 polymorphisms were predictive of response to abiraterone therapy in patients with mCRPC. Our review endorses further research in these biomarkers to serve clinical decision making for patients with mCRPC.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.01.11). Dr. Xiao Li serves as an unpaid section editor of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Buttigliero C, Tucci M, Bertaglia V. Understanding and overcoming the mechanisms of primary and acquired resistance to abiraterone and enzalutamide in castration resistant prostate cancer. Cancer Treat Rev 2015;41:884-92. [Crossref] [PubMed]
- Damber JE, Aus G. Prostate Cancer. Lancet 2008;371:1710-21. [Crossref] [PubMed]
- James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”:data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 2015;67:1028-38. [Crossref] [PubMed]
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med 2015;373:737-46. [Crossref] [PubMed]
- American Cancer Society: Cancer Facts and Figures 2012. Atlanta, Ga: American Cancer Society, 2013. Also available online. Last accessed March 4, 2014. Available online: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancerfacts-figures-2013. Last accessed March 4, 2014.
- O’Donnell A, Judson I, Dowsett M, et al. Hormonal impact of the 17alpha-hydroxylase/C (17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer 2004;90:2317-25. [Crossref] [PubMed]
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 2011;32:81-151. [Crossref] [PubMed]
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [Crossref] [PubMed]
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152-60. [Crossref] [PubMed]
- Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure andcardiovascular disease risk. Nature 2011;478:103-9. [Crossref] [PubMed]
- Wang F, Zou YF, Feng XL, et al. CYP17 gene polymorphisms and prostate cancer risk:A meta-analysis based on 38 independent studies. Prostate 2011;71:1167-77. [Crossref] [PubMed]
- Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res 2011;17:5913-25. [Crossref] [PubMed]
- Salvi S, Casadio V, Conteduca V, et al. Circulating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abiraterone. Br J Cancer 2015;112:1717-24. [Crossref] [PubMed]
- Binder M, Zhang BY, Hillman DW, et al. Common Genetic Variation in CYP17A1 and Response to Abiraterone Acetate in Patients with Metastatic Castration-Resistant Prostate Cancer. Int J Mol Sci 2016;17. [PubMed]
- Salvi S, Casadio V, Burgio SL, et al. CYP17A1 polymorphisms and clinical outcome of castration-resistant prostate cancer patients treated with abiraterone. Int J Biol Markers 2016;31:e264-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Li Z, Bishop AC, Alyamani M, et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 2015;523:347-51. [Crossref] [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [Crossref] [PubMed]
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983-92. [Crossref] [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [Crossref] [PubMed]
- Rathkopf DE, Smith MR, de Bono JS, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66:815-25. [Crossref] [PubMed]
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapynaive men with metastatic castrationresistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152-60. [Crossref] [PubMed]
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med 2017;377:352-60. [Crossref] [PubMed]
- Kang M, Jeong CW, Kwak C, et al. Comparing the clinical efficacy of abiraterone acetate, enzalutamide, and orteronel in patients with metastatic castration-resistant prostate cancer by performing a network meta-analysis of eight randomized controlled trials. Oncotarget 2017;8:59690-7. [Crossref] [PubMed]
- Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983-92. [Crossref] [PubMed]
- Roviello G, Sigala S, Danesi R, et al. Incidence and relative risk of adverse events of special interest in patients with castration resistant prostate cancer treated with CYP-17 inhibitors: A meta-analysis of published trials. Crit Rev Oncol Hematol 2016;101:12-20. [Crossref] [PubMed]
- Antonarakis ES, Lu C, Wang HN, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. [Crossref] [PubMed]
- Mitsiades N, Sung CC, Schultz N, et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res 2012;72:6142-52. [Crossref] [PubMed]
- Bellmunt J, Kheoh T, Yu MK, et al. Prior endocrine therapy impact on abiraterone acetate clinical efficacy in metastatic castration-resistant prostate cancer: Post-hoc analysis of randomised phase 3 studies. Eur Urol 2016;69:924-32. [Crossref] [PubMed]
- Friedlander TW, Roy R, Tomlins SA, et al. Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res 2012;72:616-25. [Crossref] [PubMed]
- Stranger BE, Nica AC, Forrest MS, et al. Populaton genomics of human gene expression. Nat Genet 2007;39:1217-24. [Crossref] [PubMed]
- Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patents with castration-resistant prostate cancer. Cancer Res 2009;69:2912-8. [Crossref] [PubMed]
- Diver LA, MacKenzie SM, Fraser R, et al. Common polymorphisms at the CYP17A1 locus associate with steroid phenotype: Support for blood pressure genome-wide association study signals at this locus. Hypertension 2016;67:724-32. [Crossref] [PubMed]
Cite this article as: Zhou X, Zheng Y, Zhang J, Zhang C, Jiang G, Sun S, Li X, Carrie C, Shariat SF, Lopez DS, Nielsen TK; AME Urology Disease Collaborative Group. Association between CYP17A1 polymorphisms and response to abiraterone in patients with metastatic castration-resistant prostate cancer: a systematic review. AME Med J 2018;3:50.