
Endobronchial ultrasound bronchoscopy: current uses, innovations and future directions
Introduction
Ultrasound has thoroughly transformed modern point of care medicine in many fields. In the field of chest medicine, the ability to perform ultrasound inside the thorax via the airways and esophagus has fundamentally changed the approach to certain types of chest disease. With new minimally invasive options, intrathoracic ultrasound is being used in applications ranging from less invasive staging of the mediastinum in the setting of cancer, to targeted delivery of chemotherapeutic agents. In this review we will discuss the traditional use of radial probe endobronchial ultrasound (RP-EBUS), convex probe endobronchial ultrasound (CP-EBUS) as well as the innovative ways endobronchial ultrasonography (EBUS) is being used and the future directions of EBUS for diagnosis and treatment of thoracic disease.
RP-EBUS
The radial ultrasound probe became commercially available in the 1990’s and provides a 360-degree ultrasound view of the airway and structures external to the airway (1,2). In its earliest iterations a balloon on the tip of the radial probe was used to oppose the airway wall and allow for, among other things, evaluation of extent of tumor infiltration into the airway (3). In a study of 131 patients with central thoracic malignancy, RP-EBUS was able to reliably distinguish between compression of the airway versus airway involvement with superior sensitivity to chest CT (4). The balloon tipped radial probe was also used to identify the location of mediastinal and hilar lymph nodes. After the node was located, the probe was removed and conventional transbronchial needle aspiration (TBNA) was done at the confirmed site. While this advance in EBUS technology resulted in improvement in yield of TBNA for lymph node staging (5), it was somewhat cumbersome to use in this context given that it needed to be removed and exchanged for the sampling instrument such as a needle. Though the balloon tip radial probe is utilized less in contemporary interventional pulmonology, RP-EBUS has continued to evolve in the management of chest disease.
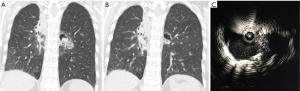
The miniaturization of the radial probe has allowed for imaging of peripheral lesions beyond the segmental airways. In this capacity, the ultrasound probe, albeit typically without a balloon, can be placed through the working channel of the bronchoscope and extended into the periphery to identify intrapulmonary nodules (6). Utilization of this technique has evolved in several ways. The radial probe can be extended through the bronchoscope to identify the lesion, leaving the bronchoscope in the appropriate subsegment and replacing the probe with biopsy tools. The addition of a guide sheath may improve diagnostic yield (7). The radial probe can be directed to the nodule via a sheath catheter and after the probe has been removed biopsy of the nodule can be done with or without fluoroscopy through the catheter that has been directed to the nodule. Using the latter technique, diagnostic yield for peripheral pulmonary nodules 2–3 cm can be as high as 72%, at single centers versus yields as low as 20% for bronchoscopy alone (7,8). If a concentric view is obtained, where the nodule encircles the probe, the diagnostic yield improves to 84% and has similar, if not improved yield, compared to computed tomography (CT) guided biopsy, with fewer complications (Figure 1) (7,9). When RP-EBUS has been combined with electromagnetic navigational bronchoscopy (ENB) the diagnostic yield for peripheral nodules is increased versus either technique alone (10,11). TBNA of the peripheral pulmonary nodule can also improve diagnostic yield by nearly 10% versus brush biopsy or forceps biopsy (12).
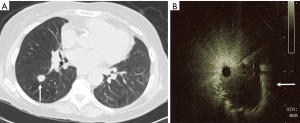
Further, imaging features noted on RP-EBUS have also been used to help risk stratify pulmonary nodules as malignant versus nonmalignant. In a study of 69 patients with peripheral pulmonary lesions undertaken by a group of experienced RP-EBUS practitioners, RP-EBUS was used to classify lesions into one of three categories based on ultrasound appearance: homogenous pattern, hyperechoic dots and linear arcs pattern, and heterogeneous pattern. Benign disease was most common in patients with homogenous pattern nodules (92%), while the majority of patients with hyperechoic dots and linear arcs pattern or heterogeneous pattern had malignant disease (99%) (6).
Recently RP-EBUS has also played a role in treatment of malignancy. RP-EBUS has been used to determine depth of tumor invasion, proximity to vasculature and other tumor characteristics in patients undergoing therapeutic bronchoscopy. In a study of 1,174 cases where RP-EBUS was used to evaluate endobronchial lesions, therapy changed in 43% of cases based on ultrasound findings (13). RP-EBUS has also been used to guide decisions regarding surgery, radiotherapy or photodynamic therapy for centrally located lung cancers (14,15). With the advent of stereotactic body radiation therapy (SBRT) for treatment of early stage non-small cell lung cancer (NSCLC), RP-EBUS has also been employed to deliver fiducial markers to guide radiotherapy (16,17).
The major limitation of RP-EBUS is the inability to perform real-time ultrasound guided procedures. Even with the use of a guide sheath to direct sampling after identification of the lesion, positioning can be lost, and sampling error can occur.
Convex probe endobronchial ultrasound
The CP-EBUS allows for real-time ultrasound guided tissues sampling. The CP-EBUS incorporates a convex transducer at the tip of a flexible bronchoscope to provide an ultrasound image that is parallel to the insertion direction of the bronchoscope (18). Some incorporate a saline filled balloon on the tip to improve approximation with the airway, while others utilize direct contact by the probe. A Doppler mode allows for assessment of vasculature and an instrument channel permits biopsy under direct visualization (Figure 2).
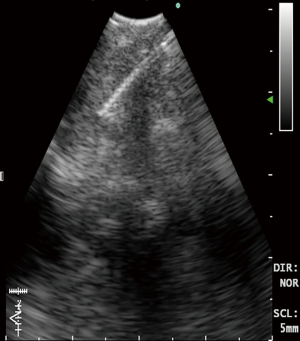
CP-EBUS is now the recommended initial diagnostic modality for mediastinal lymph node staging of lung cancer (19,20). When compared to CT and PET for lymph node staging EBUS with real time TBNA has demonstrated higher sensitivity (76.9%, 80.0%, and 92.3% respectively) and specificity (55.3%, 70.1%, and 100% respectively) (21). Prior to the advent of CP-EBUS, mediastinoscopy was the predominant technique used for mediastinal staging. Several studies have compared EBUS-TBNA with mediastinoscopy and have found equivalent diagnostic sensitivity, accuracy and NPV with fewer complications (22-24). In one comparison CP-EBUS had higher diagnostic yield, sensitivity, specificity and negative predictive value versus mediastinoscopy (91%, 87%, 100%, and 78% vs. 78%, 68%, 100%, and 59%, respectively) (24). CP-EBUS can access mediastinal and hilar lymph nodes with improved site selection and reduced sampling errors versus mediastinoscopy or conventional transbronchial needle aspiration (TBNA) although stations 5, 6, 8 and 9 are not readily accessible (25-27). With appropriate expertise the same convex probe equipped bronchoscope can be used to perform transesophageal endoscopic ultrasound guided biopsy (EUS) to reach posterior and inferior mediastinal nodes and may provide the most complete approach to minimally invasive mediastinal staging when combined with EBUS-TBNA (28,29). The diagnostic yield of EBUS-TBNA for mediastinal staging of lung cancer appears to be similar across community and academic settings (23). In addition, EBUS-TBNA appears to be more cost effective than mediastinoscopy for lymph node staging in part because of lower need for post procedural hospitalization, but also because EBUS-TBNA can be performed in the endoscopy suite as opposed to the operating room (30,31).
CP-EBUS is also useful following treatment of NSCLC when there is concern for recurrence or disease progression and has started to replace redo mediastinoscopy (32). Repeat mediastinoscopy after prior mediastinoscopy, surgical dissection or neoadjuvant therapy is complicated by adhesions and fibrosis that increase complications while decreasing diagnostic yield (33,34). EBUS-TBNA has been evaluated in patients with recurrence postoperatively with excellent sensitivity and specificity for redo staging (32,35).
In addition to lymph node sampling, CP-EBUS can be used to diagnose nodules and masses that abut the mediastinum and proximal airways. In a study of 140 patients with mediastinal masses EBUS-TBNA was diagnostic in 131 patients with no complications (36). Biopsy of peripheral lung tumors is limited by the outer diameter of the CP-EBUS bronchoscope and in most cases cannot extend beyond the lobar bronchus, though basilar segmental bronchi may be accessed in the lower lobes of some patients (18).
With the identification of driver genetic mutations as therapeutic targets in the treatment of NSCLC, the need for adequate tissue samples to facilitate testing for multiple mutations has arisen. In a study of 126 samples obtained via EBUS-TBNA, mutation analysis was performed for epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma viral oncogene (KRAS) with success rates of 97% and 93%, respectively (37). Additional studies have validated adequate tumor and lymph node tissue acquisition using EBUS-TBNA for identifying a variety of molecular alterations in non-small cell lung cancer (38-40).
Fiducial placement in central lesions using CP-EBUS has also been described (41). In this technique the transbronchial needle can be loaded with the fiducial marker with the stylet removed and the stylet can be used to deploy the marker under direct visualization. Several variations on this technique have been described using CP-EBUS to deploy fiducial markers (41-43).
Though CP-EBUS has been most often described in the diagnosis and staging of lung cancer, it also plays a significant role in the diagnosis of mediastinal adenopathy and other disease entities such as sarcoidosis, lymphoma and infectious disease. EBUS-TBNA has been used for the evaluation of mediastinal adenopathy without a primary lung cancer with reported diagnostic yield as high as 92% (44). EBUS-TBNA is commonly used for evaluation of adenopathy in the setting of suspected sarcoid and in a study of 50 patients had a diagnostic yield of 83.3% (45). There has been concern in the past that EBUS-TBNA would yield insufficient tissue for diagnosis of lymphoma based on mediastinal and hilar lymph nodes sampling, however, Moonim et al. conducted a prospective study of 100 patients with suspected lymphoma and in de novo lymphoma diagnosis was made in 88% of cases, while relapse was diagnosed in 100% of cases (46). Sensitivity for subtyping was highest in low-grade non-Hodgkins lymphoma and was lowest in Hodgkin’s disease (100% and 79% respectively). Others have supported that the reliability of EBUS-TBNA for diagnosis of lymphoma is influenced by subtype with much lower diagnostic yields in the setting of follicular and marginal zone lymphoma (47-49). Transbronchial forceps biopsy through the prior TBNA insertion site may improve diagnostic yield in these situations and can be done with real-time EBUS guidance (48). EBUS-TBNA has been employed to differentiate malignant from infectious disease and has been particularly useful in areas with high incidence of tuberculous mediastinal lymphadenitis and histoplasmosis (50-53).
The primary limitation of CP-EBUS is the diameter of the bronchoscope and inability to reach peripheral airways as previously mentioned. The endobronchial image may also be lower quality resolution in some instances compared to white light bronchoscopy without EBUS.
Complications and contraindications
The complications associated with RP-EBUS are primarily associated with biopsy and therefore are akin to transbronchial biopsy without RP-EBUS. Pneumothorax and bleeding occur at similar rates of 0.8–4.2% and 0–5.6% respectively, though significant hemorrhage may be lower in RP-EBUS with use of a guide sheath due to what is thought to be the tamponade effect of the sheath (54-56). Infection is a rare complication reported in the use of RP-EBUS (54). Compared to CT guided needle biopsy of peripheral pulmonary nodules, RP-EBUS guided biopsy has significantly lower complication rates (27% vs. 3%) (57).
Due to the rigid tip of the CP-EBUS bronchoscope, mucosal injury can occur and full thickness bronchial disruption has been reported (58). Outside of this rare complication, the majority of complications as reported in a multicenter study involving 7,345 cases are associated with biopsy, with hemorrhage being the most common complication (0.68% of cases) followed by infection (mediastinitis, pneumonia, pericarditis, cyst infection; 0.19%) and pneumothorax (0.03%). In the same study needle breakage was a complication reported in 0.20% of cases and can be prevented by avoiding excessive bending and torque on the needle (59).
The contraindications to RP-EBUS and CP-EBUS are similar to that of bronchoscopy. In non-emergent cases, bronchoscopy should be delayed 6 weeks in the setting of recent myocardial infarction, decompensated heart failure, life threatening arrhythmia, or exacerbation of asthma or chronic obstructive pulmonary disease (60). Patients should be healthy enough to undergo general anesthesia or conscious sedation for the procedure and should not have significant hemodynamic instability or hypoxemia prior to procedure. Anticoagulants and antiplatelets should be held as appropriate based on the planned procedure. In general, clopidogrel should be held 5 to 7 days prior to TBNA, endobronchial or transbronchial biopsy (61,62). Aspirin is generally considered safe to continue during TBNA and TBBX (63). INR should be <1.5 in patients on warfarin and bridging with low molecular weight heparin (LMWH) should be considered in high risk conditions, with LMWH dose held on the day of the procedure (60). The timing for discontinuing direct oral anticoagulants prior to bronchoscopy with biopsy is based on the specific drug and renal function of the patient (64). Other relative contraindications include severe pulmonary hypertension, elevated intracranial pressure, bulky anterior mediastinal masses and pregnancy (60).
New frontiers and future investigation in EBUS
There are many novel ways EBUS is being used in the diagnosis and treatment of intrathoracic disease. We and others have combined RP- EBUS and CP-EBUS with cone beam CT to facilitate biopsy of difficult to locate central and peripheral pulmonary nodules (Figures 3,4) (65). A randomized controlled trial is currently underway to determine if this technique improves diagnostic yield (NCT02978170). For thyroid malignancy EBUS has been used for diagnosis and staging in patients with lesions not amenable to percutaneous biopsy (66). It has also been used to evaluate for airway invasion in the setting of esophageal and thyroid cancer and has higher sensitivity and specificity than magnetic resonance imaging or CT (67). EBUS may also play a role in diagnosis and treatment of cardiac disease and has been used to facilitate percardiocentesis for posterior loculated pericardial effusions (68). Among 32 patients with CT proven pulmonary embolism, EBUS was utilized in a pilot study to confirm a diagnosis of central pulmonary embolism in every patient with an average procedure time of only five minutes (69). EBUS has been utilized for bronchogenic cyst drainage in patients who were not good surgical candidates and for treatment of cyst recurrence after partial resection (70,71).
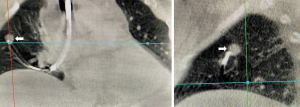
Recently, CP-EBUS been used to access lymph nodes previously thought to be reserved for mediastinoscopy. In a retrospective series, 10 cases were described where CP-EBUS was used to achieve adequate sample in 10/10 cases and diagnosis in 9/10 cases of station 5 lesions or intrapulmonary artery lesions. The authors used transpulmonary or intrapulmonary aspiration with no procedure-related complications (72).
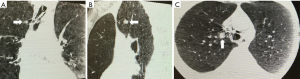
EBUS may also play a role in the direct instillation of treatment into a pulmonary lesion. EBUS transbronchial needle injection (TBNI) has been used to inject chemotherapy into pulmonary lesions and may be a therapeutic option for patients who are not candidates for systemic therapy or radiation (73,74). We recently used RP-EBUS to allow for identification of a non-resolving mycetoma in a patient with refractory hemoptysis. After localizing the lesion using RP-EBUS voriconazole and tranexamic acid were injected into the cavity via a guide sheath. On follow up imaging the mycetoma had resolved with decrease in size of the cavity and resolution of hemoptysis (Figure 5).
Several technological advances are poised to extend the application of EBUS in thoracic disease. A human feasibility study was recently done where a tracking sensor was attached to a prototype CP-EBUS bronchoscope to permit electromagnetic navigated EBUS-TBNA using preoperative CT to identify 100% of the targeted lymph nodes (75). Recently, a new thin convex probe EBUS (TCP-EBUS) has been trialed in the porcine lung and a human ex vivo lung study (76,77). The 5.9 mm tip of the TCP-EBUS, 170 degree upward angle and decreased forward oblique view (20 degrees) allowed for 22.1 mm greater maximum reach and 10.3 mm further endoscopic visibility vs. the current CP-EBUS that has a 6.9 mm tip, 135 degree upward angle and forward oblique view of 35 degrees (77). In the future the TCP-EBUS may be used to reach more peripheral pulmonary nodules and intrapulmonary lymph nodes.
Conclusions
Radial probe EBUS and convex probe EBUS continue to alter the approach to intrathoracic disease. RP-EBUS has improved the diagnostic yield of bronchoscopic peripheral pulmonary nodule biopsy. CP-EBUS is the diagnostic tool of choice for mediastinal and hilar lymph node staging of lung cancer. Both EBUS modalities have been used in a variety of novel ways to enhance the minimally invasive diagnostic and treatment capabilities of the bronchoscopist. TCP-EBUS and electromagnetic navigated EBUS are promising advances in endobronchial ultrasound that may allow for enhanced diagnostic capabilities in the future. Technological developments will likely continue to expand the application of EBUS in the diagnosis and treatment of thoracic disease.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.06.06). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. WS Krimsky is a part-time employee for the Medtronic corporation, he is also a consultant/CSO for Gala Therapeutics, consultant for Innovital systems and consultant for Peytant Solutions. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: The views reflected this manuscript are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gildea TR, Nicolacakis K. Endobronchial Ultrasound: Clinical Uses and Professional Reimbursements. Chest 2016;150:1387-93. [Crossref] [PubMed]
- Hürter T, Hanrath P. Endobronchial sonography: feasibility and preliminary results. Thorax 1992;47:565-7. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Assessment of usefulness of endobronchial ultrasonography in determination of depth of tracheobronchial tumor invasion. Chest 1999;115:1500-6. [Crossref] [PubMed]
- Herth F, Ernst A, Schulz M, et al. Endobronchial ultrasound reliably differentiates between airway infiltration and compression by tumor. Chest 2003;123:458-62. [Crossref] [PubMed]
- Herth FJ, Becker HD, Ernst A. Ultrasound-guided transbronchial needle aspiration: an experience in 242 patients. Chest 2003;123:604-7. [Crossref] [PubMed]
- Kurimoto N, Murayama M, Yoshioka S, et al. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest 2002;122:1887-94. [Crossref] [PubMed]
- Chen A, Chenna P, Loiselle A, et al. Radial probe endobronchial ultrasound for peripheral pulmonary lesions. A 5-year institutional experience. Ann Am Thorac Soc 2014;11:578-82. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic Yield of Fiberoptic Bronchoscopy in Evaluating Solitary Pulmonary Nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Ng YL, Patsios D, Roberts H, et al. CT-guided percutaneous fine-needle aspiration biopsy of pulmonary nodules measuring 10 mm or less. Clin Radiol 2008;63:272-7. [Crossref] [PubMed]
- Ozgul G, Cetinkaya E, Ozgul MA, et al. Efficacy and safety of electromagnetic navigation bronchoscopy with or without radial endobronchial ultrasound for peripheral lung lesions. Endosc Ultrasound 2016;5:189-95. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Ost DE, Ernst A, Lei X, et al. Diagnostic Yield and Complications of Bronchoscopy for Peripheral Lung Lesions. Results of the AQuIRE Registry. Am J Respir Crit Care Med 2016;193:68-77. [Crossref] [PubMed]
- Herth F, Becker HD, LoCicero J 3rd, et al. Endobronchial ultrasound in therapeutic bronchoscopy. Eur Respir J 2002;20:118-21. [Crossref] [PubMed]
- Miyazu Y, Miyazawa T, Kurimoto N, et al. Endobronchial ultrasonography in the assessment of centrally located early-stage lung cancer before photodynamic therapy. Am J Respir Crit Care Med 2002;165:832-7. [Crossref] [PubMed]
- Takahashi H, Handa M, Oyaizu A, et al. Kyobu Geka 2001;54:907-12. [Ultrasonographical approach for the diagnosis on the depth of invasion in early bronchogenic squamous cell carcinoma]. [PubMed]
- Steinfort DP, Siva S, Kron T, et al. Multimodality guidance for accurate bronchoscopic insertion of fiducial markers. J Thorac Oncol 2015;10:324-30. [Crossref] [PubMed]
- Obstoy B, Lachkar S, Salaun M, et al. Assessment of radial EBUS-GS for disposition of fiducial gold marker in peripheral lung nodule before stereotaxic radiation therapy. Eur Respir J 2014;44:690.
- Yasufuku K, Nakajima T, Chiyo M, et al. Endobronchial ultrasonography: current status and future directions. J Thorac Oncol 2007;2:970-9. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7s-37s.
- Yasufuku K, Nakajima T, Motoori K, et al. Comparison of endobronchial ultrasound, positron emission tomography, and CT for lymph node staging of lung cancer. Chest 2006;130:710-8. [Crossref] [PubMed]
- Um SW, Kim HK, Jung SH, et al. Endobronchial ultrasound versus mediastinoscopy for mediastinal nodal staging of non-small-cell lung cancer. J Thorac Oncol 2015;10:331-7. [Crossref] [PubMed]
- Warren WA, Hagaman JT. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Mediastinal Staging in a Community Medical Center. Ann Am Thorac Soc 2016;13:1802-7. [PubMed]
- Ernst A, Anantham D, Eberhardt R, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577-82. [Crossref] [PubMed]
- Jalil BA, Yasufuku K, Khan AM. Uses, limitations, and complications of endobronchial ultrasound. Proceedings (Baylor University Medical Center) 2015;28:325-30. [Crossref] [PubMed]
- Jiang J, Browning R, Lechtzin N, et al. TBNA with and without EBUS: a comparative efficacy study for the diagnosis and staging of lung cancer. J Thorac Dis 2014;6:416-20. [PubMed]
- Yasufuku K, Pierre A, Darling G, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg 2011;142:1393-400.e1. [Crossref] [PubMed]
- Wallace MB, Pascual JM, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. Jama 2008;299:540-6. [Crossref] [PubMed]
- Dhooria S, Aggarwal AN, Gupta D, et al. Utility and Safety of Endoscopic Ultrasound With Bronchoscope-Guided Fine-Needle Aspiration in Mediastinal Lymph Node Sampling: Systematic Review and Meta-Analysis. Respir Care 2015;60:1040-50. [Crossref] [PubMed]
- Czarnecka-Kujawa K, Yasufuku K. The role of endobronchial ultrasound versus mediastinoscopy for non-small cell lung cancer. J Thorac Dis 2017;9:S83-97. [Crossref] [PubMed]
- Navani N, Lawrence DR, Kolvekar S, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med 2012;186:255-60. [Crossref] [PubMed]
- Yamamoto T, Sakairi Y, Nakajima T, et al. Comparison between endobronchial ultrasound-guided transbronchial needle aspiration and 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of postoperative nodal recurrence in patients with lung cancer. Eur J Cardiothorac Surg 2015;47:234-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. [Crossref] [PubMed]
- Van Schil P, van der Schoot J, Poniewierski J, et al. Remediastinoscopy after neoadjuvant therapy for non-small cell lung cancer. Lung Cancer 2002;37:281-5. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Yasufuku K, Nakajima T, Fujiwara T, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal masses of unknown etiology. Ann Thorac Surg 2011;91:831-6. [Crossref] [PubMed]
- Stigt JA. Pyrosequencing analysis of EGFR and KRAS mutations in EUS and EBUS-derived cytologic samples of adenocarcinomas of the lung. J Thorac Oncol 2013;8:1012-8. [Crossref] [PubMed]
- Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of Lymph Node Transbronchial Needle Aspirates Using Convex Probe Endobronchial Ultrasound for Multiple Tumor Genotyping Techniques in Non–Small-Cell Lung Cancer. J Thorac Oncol 2013;8:1438-44. [Crossref] [PubMed]
- Righi L, Franzi F, Montarolo F, et al. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)—from morphology to molecular testing. J Thorac Dis 2017;9:S395-404. [Crossref] [PubMed]
- Oezkan F, Khan AM, Zarogoulidis P, et al. Efficient utilization of EBUS-TBNA samples for both diagnosis and molecular analyses. Onco Targets Ther 2014;7:2061-5. [PubMed]
- Argento AC, Decker R, Puchalski J. Fiducial Marker Placement Via Convex Probe EBUS. J Bronchology Interv Pulmonol 2016;23:181-5. [Crossref] [PubMed]
- McGuire FR, Liming J, Ochran T, et al. Real-time Endobronchial Ultrasound-guided Implantation of Radiotherapy Monitoring Devices. J Bronchology Interv Pulmonol 2007;14:59-62.
- Harris K, Gomez J, Dhillon SS, et al. Convex probe endobronchial ultrasound placement of fiducial markers for central lung nodule (with video). Endosc Ultrasound 2015;4:156-7. [Crossref] [PubMed]
- Gahlot T, Parakh U, Verma K, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in diagnosing mediastinal lymphadenopathy. Lung India 2017;34:241-6. [PubMed]
- Tremblay A, Stather DR, MacEachern P, et al. A Randomized Controlled Trial of Standard vs Endobronchial Ultrasonography-Guided Transbronchial Needle Aspiration in Patients With Suspected Sarcoidosis. Chest 2009;136:340-6. [Crossref] [PubMed]
- Moonim MT, Breen R, Fields PA, et al. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216-23. [Crossref] [PubMed]
- Farmer PL, Bailey DJ, Burns BF, et al. The reliability of lymphoma diagnosis in small tissue samples is heavily influenced by lymphoma subtype. Am J Clin Pathol 2007;128:474-80. [Crossref] [PubMed]
- Kheir F, Itani A, Assasa O, et al. The utility of endobronchial ultrasound-transbronchial needle aspiration in lymphoma. Endosc Ultrasound 2016;5:43-8. [Crossref] [PubMed]
- Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804-9. [Crossref] [PubMed]
- Navani N, Molyneaux PL, Breen RA, et al. Utility of endobronchial ultrasound-guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889-93. [Crossref] [PubMed]
- Eom JS, Mok JH, Lee MK, et al. Efficacy of TB-PCR using EBUS-TBNA samples in patients with intrathoracic granulomatous lymphadenopathy. BMC Pulm Med 2015;15:166. [Crossref] [PubMed]
- Egressy KvL. Mohammed M, Ferguson JS. The Use of Endobronchial Ultrasound in the Diagnosis of Subacute Pulmonary Histoplasmosis. Diagn Ther Endosc 2015;2015:510863 [PubMed]
- del Castillo M, Rajmane R, Schenck E. A Novel Application of Endobronchial Ultrasound Guided Fine Needle Aspiration to Diagnose Lymphatic Cryptococcosis in HIV Positive Host. Chest 2012;142:893A. [Crossref]
- Hayama M, Izumo T, Matsumoto Y, et al. Complications with Endobronchial Ultrasound with a Guide Sheath for the Diagnosis of Peripheral Pulmonary Lesions. Respiration 2015;90:129-35. [Crossref] [PubMed]
- Huang CT, Ruan SY, Liao WY, et al. Risk factors of pneumothorax after endobronchial ultrasound-guided transbronchial biopsy for peripheral lung lesions. PLoS One 2012;7:e49125 [Crossref] [PubMed]
- Zhang L, Wu H, Wang G. Endobronchial ultrasonography using a guide sheath technique for diagnosis of peripheral pulmonary lesions. Endosc Ultrasound 2017;6:292-9. [Crossref] [PubMed]
- Steinfort DP, Vincent J, Heinze S, et al. Comparative effectiveness of radial probe endobronchial ultrasound versus CT-guided needle biopsy for evaluation of peripheral pulmonary lesions: A randomized pragmatic trial. Respir Med 2011;105:1704-11. [Crossref] [PubMed]
- Sehgal R, Branca P, Sheikh N. Bronchial Tear Associated With Convex Probe Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: An Unusual Complication. Chest 2017;152:A872. [Crossref]
- Asano F, Aoe M, Ohsaki Y, et al. Complications associated with endobronchial ultrasound-guided transbronchial needle aspiration: a nationwide survey by the Japan Society for Respiratory Endoscopy. Respir Res 2013;14:50. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Ernst A, Eberhardt R, Wahidi M, et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest 2006;129:734-7. [Crossref] [PubMed]
- Pathak V, Allender JE, Grant MW. Management of anticoagulant and antiplatelet therapy in patients undergoing interventional pulmonary procedures. Eur Respir Rev 2017;26:170020 [Crossref] [PubMed]
- Youness HA, Keddissi J, Berim I, et al. Management of oral antiplatelet agents and anticoagulation therapy before bronchoscopy. J Thorac Dis 2017;9:S1022-33. [Crossref] [PubMed]
- Lange CM, Fichtlscherer S, Miesbach W, et al. The Periprocedural Management of Anticoagulation and Platelet Aggregation Inhibitors in Endoscopic Interventions. Deutsches Ärzteblatt International 2016;113:129-35. [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Vogl T, et al. Cone Beam Computertomography (CBCT) in Interventional Chest Medicine - High Feasibility for Endobronchial Realtime Navigation. J Cancer 2014;5:231-41. [Crossref] [PubMed]
- Steinfort DP, Irving LB. Endobronchial ultrasound staging of thyroid lesion in small cell lung carcinoma. Thorac Cardiovasc Surg 2010;58:128-9. [Crossref] [PubMed]
- Wakamatsu T, Tsushima K, Yasuo M, et al. Usefulness of Preoperative Endobronchial Ultrasound for Airway Invasion around the Trachea: Esophageal Cancer and Thyroid Cancer. Respiration 2006;73:651-7. [Crossref] [PubMed]
- Sharma RK, Khanna A, Talwar D. Endobronchial Ultrasound: A New Technique of Pericardiocentesis in Posterior Loculated Pericardial Effusion. Chest 2016;150:e121-3. [Crossref] [PubMed]
- Aumiller J, Herth FJF, Krasnik M, et al. Endobronchial Ultrasound for Detecting Central Pulmonary Emboli: A Pilot Study. Respiration 2009;77:298-302. [Crossref] [PubMed]
- Dhand S, Krimsky W. Bronchogenic cyst treated by endobronchial ultrasound drainage. Thorax 2008;63:386. [Crossref] [PubMed]
- Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst's recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg 2006;29:627-9; discussion 9. [Crossref] [PubMed]
- Mehta RM, Biraris PR, Pattabhiraman V, et al. Defining expanded areas in EBUS sampling: EBUS guided trans- and intra-pulmonary artery needle aspiration, with review of transvascular EBUS. Clin Respir J 2018;12:1958-63. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer 2015;90:542-7. [Crossref] [PubMed]
- Sorger H, Hofstad EF, Amundsen T, et al. A multimodal image guiding system for Navigated Ultrasound Bronchoscopy (EBUS): A human feasibility study. PLoS One 2017;12:e0171841 [Crossref] [PubMed]
- Wada H, Hirohashi K, Nakajima T, et al. Assessment of the New Thin Convex Probe Endobronchial Ultrasound Bronchoscope and the Dedicated Aspiration Needle: A Preliminary Study in the Porcine Lung. J Bronchology Interv Pulmonol 2015;22:20-7. [Crossref] [PubMed]
- Patel P, Wada H, Hu HP, et al. First Evaluation of the New Thin Convex Probe Endobronchial Ultrasound Scope: A Human Ex Vivo Lung Study. Ann Thorac Surg 2017;103:1158-64. [Crossref] [PubMed]
Cite this article as: Warren WA, Sobieszczyk MJ, Sarkar S, Krimsky WS. Endobronchial ultrasound bronchoscopy: current uses, innovations and future directions. AME Med J 2018;3:70.

