Management of benign central airway obstruction
Introduction
Benign central airway obstruction (CAO) is defined as a non-malignant disease process resulting in narrowing of the trachea and main stem bronchi. While the term “benign” CAO is used to describe all types of airway obstruction caused by diseases other than malignancy, it is often associated with significant morbidity and mortality. There are a multitude of benign processes that can result in critical airway stenosis (Table 1).
Table 1
| Post-traumatic: |
| Post-intubation tracheal injury |
| Post-tracheostomy tracheal injury |
| Stent-related tracheal and bronchial stenosis |
| Granulation tissue from foreign bodies |
| Inhalational injury |
| Radiation |
| Airway trauma |
| Inflammatory airway disease: |
| Granulomatosis with polyangiitis |
| Amyloidosis |
| Sarcoidosis |
| Relapsing polychondritis |
| Idiopathic laryngotracheal stenosis |
| Benign endobronchial tumors: |
| Squamous cell papilloma |
| Papillomatosis |
| Hamartoma |
| Leiomyoma |
| Lipoma |
| Fibroma |
| Neurogenic tumors |
| Pleomorphic adenoma |
| Mucus gland adenoma |
| Oncocytoma |
| Tracheobronchopathia osteochondroplastica |
| Lymphadenopathy |
| Broncholithiasis |
| Fibrosing mediastinitis |
| Thyroid disease |
| Goiter |
| Cysts |
| Thyroiditis |
| Vascular |
| Right aortic arch |
| Double aortic arch |
| Pulmonary artery sling |
| Left carotid artery anomaly |
| Aortic aneurysm |
| Mediastinal cysts |
| Chest wall and spinal deformities |
| Kyphoscoliosis |
| Pectus excavatum |
| Straight back syndrome |
| Infections: |
| Viral |
| Bacterial |
| Mycobacterial |
| Fungal |
| Parasites |
| Dynamic expiratory narrowing: |
| Tracheobronchomalacia |
| Excessive dynamic airway collapse |
| Post-surgical causes of airway narrowing: |
| Surgical anastomosis |
| Post-pneumonectomy syndrome |
This article will provide an overview of the etiology, diagnosis and management of several causes of benign airway obstruction including post-intubation and tracheostomy stenosis, inflammatory disorders, benign endobronchial tumors, airway obstruction related to infections, and extrinsic compression of the airway by non-malignant processes. Other causes of benign CAO including tracheobronchomalacia, stenosis associated with lung transplant, and airway foreign bodies are covered elsewhere in this issue.
Mechanisms of obstruction
Non-malignant processes can narrow the central airway through either intraluminal obstruction of the airway or by extrinsic compression of the airway. Benign CAO may also occur with cartilaginous malacia involving segmental or diffuse weakness of the tracheal and bronchial walls and excessive dynamic airway collapse associated with pronounced invagination of the posterior membrane (1).
Presentation
The clinical presentation of benign CAO is often indistinguishable from that of malignant airway narrowing, and includes dyspnea, cough, hemoptysis, wheezing and stridor. The location, rate, and extent of the narrowing as well as the patient’s cardiopulmonary reserve will influence the type and severity of symptoms (2). Given the non-specific nature of these symptoms, the diagnosis of CAO is often delayed, and the patient misdiagnosed with more common disorders such as asthma and chronic obstructive pulmonary disease (COPD).
In cases of concomitant systemic disease such as inflammatory and infectious disorders, the presentation may include a variety of systemic symptoms. The onset of symptoms can be sudden, as in the case of a foreign body aspiration or a retained mucus plug at the site of a fixed stenosis, or gradual as may be seen with slow-growing benign tumors.
With respect to fixed tracheal stenoses, patients typically become dyspneic with exertion when the diameter has narrowed to less than 8 mm and experience dyspnea at rest when the diameter is less than 5 mm, though this may vary based on cardiopulmonary reserve (2). In cases of dynamic airway compromise such as tracheobronchomalacia, the onset of symptoms may be more variable. Given the relatively late onset of symptoms, up to 54% of patients with tracheal stenosis present with respiratory distress (3).
Diagnostic evaluation
Imaging
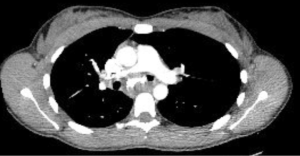
Chest radiography, typically the first imaging study obtained in a patient with respiratory symptoms, is relatively insensitive and may be interpreted as normal even in the cases of critical airway narrowing. Chest CT is the imaging modality of choice and can discern the presence of an endoluminal lesion, compression of the airway by an extrinsic lesion, mucoid impaction, distal atelectasis, air trapping, and post-obstructive pneumonia (4). Multi-detector chest CTs with high resolution, contrast enhancement, multiplanar reformation, volume rendering, and virtual bronchoscopy can better characterize the location, shape, and extent of central airway pathology and play a complementary role to bronchoscopy in the assessment of patients with CAO (5,6). Dynamic chest CT imaging with images obtained at end-inspiration and during breathing provide a non-invasive method to assess dynamic airway narrowing as in the case of tracheobronchomalacia and excessive dynamic airway collapse (1,7). In fact, dynamic CT has been shown to have comparable accuracy to bronchoscopy and improved sensitivity over end-expiratory CT for the diagnosis of tracheobronchomalacia (8).
Physiologic evaluation
Flow-volume loops can assist with the clinical diagnosis and monitoring of the progression of CAO. Fixed airway obstruction is typically associated with blunting of both the inspiratory and expiratory limbs of the flow-volume loop. Maximum inspiratory flow is largely decreased with variable extrathoracic obstruction, and maximum expiratory flow is decreased with variable intrathoracic obstruction (9) (Figure 1). Although informative when present, the blunting of the flow-volume loop typically does not occur until the airway is narrowed to 8–10 mm (2). Spirometry is not reliable for quantifying the degree of CAO, and it does not identify the exact location, extent, or morphology of the airway narrowing. It also has low sensitivity for detecting mild to moderate reductions in airway caliber.

Bronchoscopic assessment
Bronchoscopy is essential in the assessment of benign CAO as it provides a detailed view of the location and extent of narrowing as well as direct assessment of the change of the narrowing with respiration. Intraluminal lesions can be sampled with a variety of endoscopic tools including forceps, needles, and brushings. Endobronchial ultrasound can be used to assess airway narrowing caused by extrinsic compression. Lesions can be classified as soft tissue, vascular, or cystic, and needle aspiration can be performed for diagnosis if indicated.
The decision as to whether the patient should undergo flexible or rigid bronchoscopy depends on several factors including the nature of the lesion, the experience of the endoscopist, and the availability of staff, equipment, and facilities. Situations in which rigid bronchoscopy may be a more effective tool include critical airway narrowing, vascular airway lesions, and need for endobronchial tissue debridement. In addition, the bronchoscope can be used to bypass the obstruction and assess the patency of the distal airways, and thus help inform the proceduralist as to whether therapeutic bronchoscopic techniques including ablation, mechanical dilation, and stent placement are indicated.
Care is advised when approaching a severe airway stenosis with flexible bronchoscopy as minimal mucosal trauma with the scope could result in edema and complete airway obstruction. In these cases, it is advised that the stenosis not be traversed with the flexible scope and instead deferred until therapeutic rigid bronchoscopy is performed. Given the potential for a dire outcome if critical airway narrowing is not appropriately managed, bronchoscopy should be performed by an experienced bronchoscopist with skilled staff, appropriate equipment and surgical and anesthesia support.
Therapeutic approaches
The management of benign CAO, especially when associated with a concomitant systemic disease, requires a multidisciplinary approach, often including interventional pulmonologists, thoracic surgeons, otolaryngologists, rheumatologists, infectious disease specialists, and anesthesiologists. The principal goals of benign CAO management include airway stabilization, resolution of symptoms and improved quality of life through improving airway patency.
Several bronchoscopic modalities are currently available to treat airway obstruction including hot therapies (Nd:YAG laser, electrocautery, argon plasma coagulation), cold therapies (cryotherapy, cryodebridement, cryospray), microdebrider, balloon dilation, and airway stenting. The particular tools chosen to manage the airway narrowing depends on the nature and location of the lesion, the skills of the proceduralist, and the available equipment. In properly selected patients, stent placement for the management of mechanical ventilation and artificial airway dependency due to benign CAO can offer long-term airway patency (10).
In some cases, surgical management is preferable. This decision depends on a number of factors including the underlying disease process, the location and extent of disease, and the surgical candidacy of the patient. In selected patients with benign CAO, surgery may provide a more durable and satisfactory option than endoscopic management. Surgical options may include end-to-end anastomosis, sleeve resection, and tracheoplasty.
Management by disease process
Post-traumatic causes
Post-intubation (PITS) and post-tracheostomy (PTTS) tracheal stenosis
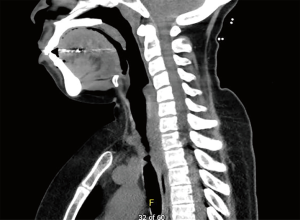
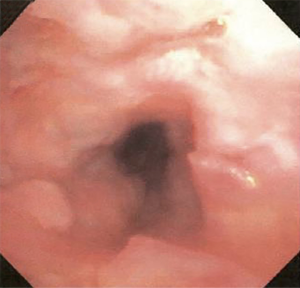
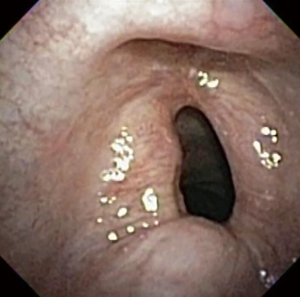
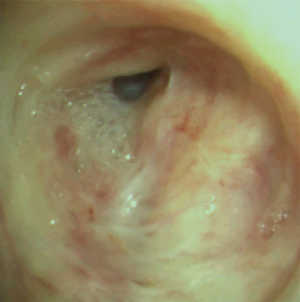
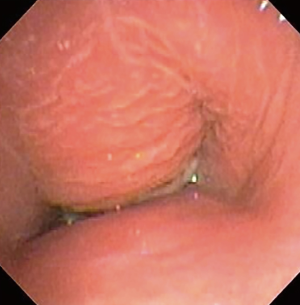
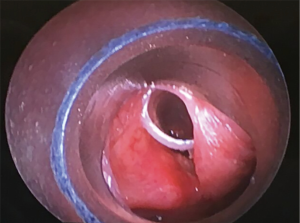
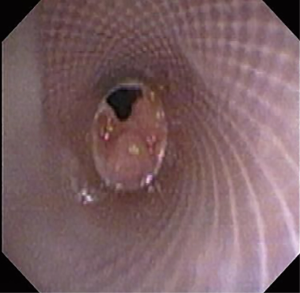
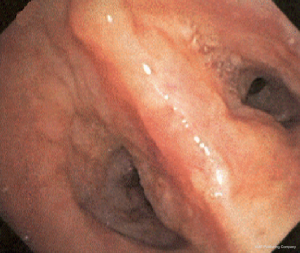
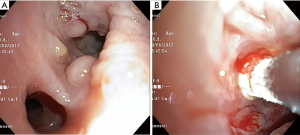
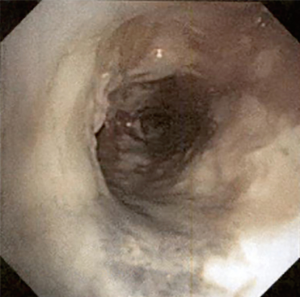
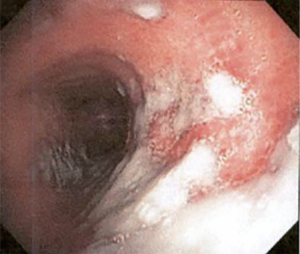
The most common causes of acquired benign tracheal stenosis are PITS (Figures 2,3) and PTTS (Figures 4-6) with an estimated incidence of 4.9 cases per million per year (11). Treatment considerations are determined by the site, severity, and type of stenosis as well as the patient’s symptoms and comorbidities. Simple stenosis is defined as a web-like, membranous concentric stenosis without damage to the cartilage and <1 cm in length (3). For simple stenoses, bronchoscopic intervention is the preferred treatment. For more complex lesions (≥1 cm in length, circumferential hourglass-like contraction scarring, or malacia), tracheal sleeve resection may be needed for a “cure”. However, in patients who are not surgical candidates, advanced bronchoscopic interventions have been demonstrated to have success rates up to 70% (12,13). Thus, a multidisciplinary approach with an initial conservative treatment strategy and with surgery reserved for recurrent stenosis may be reasonable (3).
The clinical significance of differentiating between PITS and PTTS was recently evaluated. Compared with PITS, patients with PTTS had more mixed lesions with a higher grade of tracheal stenosis located at a lower site. The success rate of bronchoscopic intervention was higher in the PITS group than in the PTTS group (76.9% vs. 63.6%, P=0.043) (14). Simple stenoses also responded better than complex stenoses (96% vs. 79%) (13).
A multimodality approach is employed to maximize success rates, most commonly including radial incisions, balloon dilatation, and stent placement. The combination of electrocautery knife radial incisions with balloon dilation resulted in greater improvement in the degree of stenosis and decreased recurrence rates compared to balloon dilation alone (15). The radial cuts are generally made at the 3, 9, and 12 o’clock position through the entire length of scar tissue at an area of stenosis. Nd:YAG laser can also be used to make the radial incisions (16). Initial studies of cryospray use followed by balloon dilation for the management of PITS and PTTS have shown success in improving symptoms and reducing the severity of airway narrowing (17,18). After dilation of the airway, silicone stents are utilized to maintain airway patency in selected cases (13). For PITS, it appears that early stenting (within 6 months of intubation) helps provide support during the remodeling phase and decreases the likelihood of scarring, thus allowing stent removal (19). The duration of stenting is individualized based on clinical symptoms and endoscopic examination.
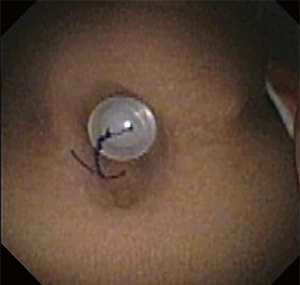
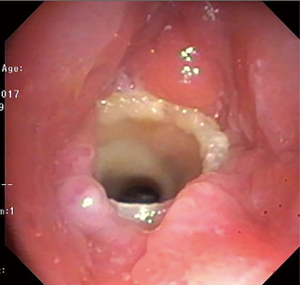
Chronic complications of stents include granulation tissue overgrowth, stent migration, mucostasis, and restenosis (14). Thus, repeat bronchoscopic interventions are necessary as often as every 1-4 months, especially in patients with complex stenoses (12). Migration of silicone stents placed in the subglottis and upper trachea have been reported in up to 17.5% of patients (20) (Figure 7). To prevent migration, the Montgomery T-tube, a silicone tube that functions as both a tracheal stent and a tracheostomy tube with an external limb to secure the device in the airway and provide a conduit for aspirating secretions, can be utilized (21). Additionally, a button to externally fix silicone stents located in the upper trachea has been described (22) (Figure 8). The use of self-expanding metallic tracheal stents is inadvisable in this condition due to granulation tissue development and impact on future surgical resection (23).
A variety of other tools are available for the management of PITS and PTTS. Microdebrider bronchoscopy can be used to rapidly remove tracheal granulation tissue from prior intubation or tracheostomy (24). The application of mitomycin C has been shown to lengthen the symptom-free period and decrease the number of repeat procedures (25,26). Recently, topical paclitaxel has been used as adjuvant treatment for cicatricial PITS and PTTS with high rates of durable remission (27).
Stent-related stenosis
Surveillance bronchoscopy within 4 to 6 weeks of stent placement is recommended for the early detection and management of complications. Sixty percent of asymptomatic patients in one study were found to have at least one stent-related complication on follow-up bronchoscopy (28). Rates of airway stent complications, including obstruction secondary to granulation tissue and restenosis, vary according to the type of stent and the indication for placement (Figures 9,10).
Rates of granulation tissue formation range up to 9% for silicone stents, 9.5% for balloon-expanding stents, 33% for Montgomery t-tubes, and 48% for self-expandable metallic stents (SEMS) (21,29-31). Most obstructive granulation tissue is detected in the first year after implantation. The time to granulation tissue formation after SEMS placement in patients with benign airway obstruction was longer than that in patients with malignant conditions (median 212 vs. 31 days; P=0.005) (32), and did not appear to be influenced by stent location, type (covered vs. uncovered), length, or site of granulation tissue (31).
Management strategies for stent restenosis or granulation tissue include electrocautery, balloon dilation, stent removal, or implantation of another stent with success in 81% of patients (32). The application of thermal therapies with stents in place can cause stent damage, including discoloration, ignition, and rupture (33). Thus, stents are removed prior to the use of endobronchial heat therapy. Granulomas can be treated with argon plasma coagulation, Nd:YAG laser, and cryotherapy (12). In one study, 28 patients with PITS underwent brachytherapy due to recurrent granulation tissue formation after stent placement. All patients experienced a reduction in therapeutic bronchoscopic procedures after brachytherapy compared with the pretreatment period, and there were no treatment-related complications (34). Sixty-six percent of patients treated with endobronchial brachytherapy for recurrent tracheal granulation tissue had no recurrence over a 36-month follow-up period (35). A novel paclitaxel-eluting tracheal stent has been shown to reduce granulation tissue formation in canine models and may be considered for potential use in humans in the future (36). Restenosis after placement of SEMS in lung transplant patients can be managed with balloon dilatation, argon plasma coagulation, cryotherapy, brachytherapy, and stent-in-stent insertion (37).
Idiopathic laryngotracheal stenosis (ILTS)
ILTS, a rare disorder of unknown etiology, is characterized by an inflammatory cicatricial stenosis involving the subglottis and upper trachea. This process almost exclusively affects women between 20–60 years of age. Exertional dyspnea and “noisy” breathing are common presenting symptoms (38). The stenosis may progress over months to years, with one study showing an average duration of symptoms of 3.1 years prior to diagnosis (39). Not uncommonly, patients are misdiagnosed with another respiratory process such as asthma. The association of gastroesophageal reflux disease (GERD) and ILTS has been suggested, with a reported prevalence of GERD in this patient population varying from 33–65% (40,41). Numerous classification systems and treatment strategies for laryngotracheal stenosis have been proposed (42). Severity and extent of airway narrowing, morphology of stenosis, and functional impairment are factors that are assessed in determining treatment of ILTS.
While surgical resection is often considered the procedure of choice in patients with ILTS, endoscopic management may be performed for simple web-like stenoses or in cases where surgery is not safe or feasible (severe comorbidities, high subglottic stenoses, long >4–6 cm stenosis). Endoscopic treatments include topical and systemic corticosteroids, mechanical dilation, hot ablative therapy, cryotherapy, mitomycin C and stent placement (18). In one study of 38 patients with ILTS managed with laser, mitomycin C or corticosteroid injection, and dilation by balloon or bougie, 21% required one procedure while 79% required multiple procedures. The time interval between endoscopic procedures did not decrease over time in this study (39). Airway stents may be utilized in patients who are not surgical candidates or as a bridge to surgery (43). As migration of subglottic stents is a common problem, options including external fixation of the stent with a neck button or use of a Montgomery T tube may be considered (22).
The optimal surgical procedure to manage ILTS remains controversial. Cartilage grafts to enlarge the laryngotracheal lumen or segmental laryngotracheal resection with primary end-to-end anastomosis have been used. In one study of 73 patients who underwent single-staged laryngotracheal resection, 91% had good to excellent long-term results with voice and breathing quality and did not require further intervention (44).
Systemic diseases
Relapsing polychondritis (RP)
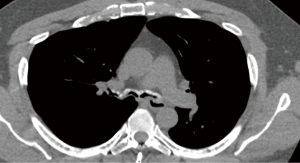
RP is a chronic multi-system autoimmune disease characterized by recurrent episodes of cartilaginous inflammation. The prevalence of airway involvement in a large cohort of patients with RP was 21%. Airway findings included subglottic stenosis, focal and diffuse malacia, and focal stenosis in different areas of the bronchial tree (45) (Figures 11,12). Dynamic CT performed to evaluate for malacia may also demonstrate airway wall thickening and calcification sparing the posterior membrane and air trapping (46,47). PET-CT has become a valuable imaging tool for the early diagnosis of RP and the evaluation of disease extent and activity during treatment (48,49).
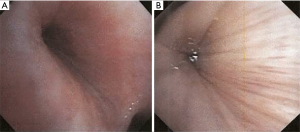
Bronchoscopic interventions are usually reserved for patients who require a bridging intervention while waiting for medical therapy to take effect, have failed medical therapy, or have severe airway stenosis or CAO. Patients who seem to benefit the most are ones with severe focal stenosis or diffuse tracheobronchomalacia (50). Forty percent of patients with airway involvement required intervention including balloon dilation, stent placement, tracheostomy, or a combination of these treatments with improvement in dyspnea symptoms. Both metallic and silicone stents have been used in RP patients (45). Silicone stent removal is typically performed 6–12 months after the stabilization of the airway. Early tracheostomy may be necessary to prevent sudden death from airway obstruction in patients with glottic or subglottic stenosis (51).
Granulomatosis with polyangiitis (GPA)
Subglottic stenosis has been reported to occur in up to 22% of patients with GPA (52). Bronchial stenosis is also a common finding and has a higher rate of restenosis than subglottic stenosis (53) (Figure 13). The recommended treatment of stenosis in GPA is the combination of intralesional injection of corticosteroids, balloon dilatation, and radial incisions. This approach has proven to be a safe and effective strategy for treating obstructive tracheobronchial GPA and can obviate the need for airway bypass or stenting (54-57). In a large case series, a 12-month period of airway stability was achieved in 97% of patients with an adverse event rate of 6.6% (54). The median intervention-free interval can range from 4 to 22 months with a median of 1–2 procedures per patient (54,55). Post-operatively, patients can be treated with a 2-week systemic steroid taper in an attempt to avoid restenosis during the re-epithelialization phase (58). In addition, a daily prednisone dose ≥30 mg at the time of endoscopic intervention was associated with a lower incidence of treatment failure (53).
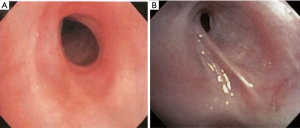
Stents are generally avoided in GPA because they are associated with increased inflammation and disease reactivation. Tracheostomies may be used for emergency situations or for severe destruction of the laryngeal or tracheal structures as a disease-related complication (59,60). Surgical resection with re-anastomosis is reserved for selected cases of severe and longer stenoses; however, many of these patients may also require subsequent airway dilation (58,59,61).
Amyloidosis
Amyloidosis is a systemic disease that involves the abnormal extracellular deposition of amyloid and fibrillar protein material in beta-pleated sheets. Tracheobronchial amyloidosis (TBA) accounts for 1.1% of all amyloidosis (62). CT imaging can demonstrate tracheal stenosis, tracheal wall thickening, calcification of the airway wall sparing the posterior membrane, atelectasis, and hilar space-occupying lesions. Bronchoscopic findings include multiple nodules or masses, luminal stenosis, friable mucosa, mucosal roughness or unevenness, and mucosal hyperemia and edema (63) (Figure 14). The amyloid deposits can range in appearance from circumscribed, superficial yellow lesions to erythematous, raised cobblestoning of the airway mucosa (62). Narrow band imaging revealing complex vascular networks, capillary loops, and abrupt-ending large caliber vessels have been described in patients with TBA (64). Histopathology of TBA lesions reveals apple-green birefringence under polarized light with Congo red staining or yellow-green birefringence under polarizing microscopy.
Treatment options include bronchoscopic recanalization, external beam radiation, and systemic drug therapy. Due to the friable nature of the amyloid-affected mucosa, excessive and even fatal bleeding has been reported during bronchoscopic biopsies in patients with TBA (65,66). The most commonly used bronchoscopic interventions are Nd:YAG laser, argon plasma coagulation, and cryotherapy (63,67). Stenting and surgical resection are rarely performed (63,68,69). External beam radiation therapy with doses of 20–24 Gy has been shown to improve clinical symptoms and FEV1 (70,71). Eight out of 10 patients had local control sustained at a median follow-up of 6.7 years (72). Amyloidosis localized to the tracheobronchial tree does not require drug therapy; however, patients with systemic amyloidosis can be treated with stem cell transplantation or melphalan-corticosteroid treatment (73).
Sarcoidosis
Sarcoidosis is a multisystem granulomatous disease characterized by the formation of noncaseous epithelioid cell granulomas. Airway involvement is initially manifested by mucosal edema, erythema, and the formation of granulomas. With disease progression, the mucosa becomes granular, nodular, and friable. Large sarcoid nodules can cause obstruction of airways, and scarring leads to stenosis and anatomic distortion. Sarcoid granulomas are characterized by waxy yellow mucosal nodules, usually 2–4 mm in diameter (74).
Endoluminal stenosis is rare, occurring in 18 out of 2,500 patients with sarcoidosis. Stenoses can be solitary or multiple and affect the mainstem, lobar, and segmental bronchi. Endobronchial involvement with or without parenchymal disease is treated similarly to systemic sarcoidosis. In one series, treatment with systemic steroids within 3 months of diagnosis was associated with symptom relief and endoscopic improvement (75). Bronchoscopic interventions can be applied for lesions that persist despite drug therapy; however, data is limited to case reports and small case series.
Balloon dilation of stenosis, with or without Nd:YAG laser photoresection, has been shown to be a safe and effective method for improving respiratory symptoms (76,77). In one case report, the addition of topical mitomycin C to balloon dilation provided only short-term relief (78). Metallic stent placement after laser photoresection and balloon dilation has been used for lobar stenosis (79). However, stenting for sarcoidosis should be reserved for patients with treatment failure, recurrence of stenosis, and cartilaginous destruction (80). Laryngeal sarcoidosis has been treated with intralesional steroid injections, tracheotomy, surgery, and low-dose external beam radiation therapy (81).
Benign endobronchial lesions
Broncholithiasis
Broncholithiasis is defined as a condition in which calcified or ossified material is present within the bronchial lumen. This condition can lead to radiographic abnormalities such as atelectasis, mucoid impaction, bronchiectasis, or expiratory air trapping (Figure 15). The most common cause of airway obstruction is erosion by a calcified adjacent lymph node into the bronchial lumen, usually associated with long-standing necrotizing granulomatous lymphadenitis (82). Based on CT chest and bronchoscopic findings, broncholiths can be classified as intraluminal, extraluminal, or mixed. Intraluminal broncholiths can be removed via bronchoscopy with high success rates, and extraluminal broncholiths are typically managed with surgery. Mixed broncholiths can be difficult to completely remove broncoscopically and, due to the close proximity to the pulmonary artery branches, non-surgical attempts increase the risk of potentially fatal hemorrhage (82-84).
Attempts to remove seven mixed broncholiths via flexible bronchoscopy in one study resulted in incomplete removal with recurrent hemoptysis in 2 patients (83). Forty-eight percent (23 of 48) of partially eroding broncholiths were successfully removed broncoscopically in another series, with a greater percentage removed with the rigid bronchoscope (67%) than with the flexible bronchoscope (33%). Hemorrhage occurred in one of these patients requiring thoracotomy (85). All free broncholiths were completely extracted regardless of the type of bronchoscope used.
Bronchoscopic tools to remove broncholiths include forceps, snares, and pulsed dye laser and Nd:YAG laser to fragment the broncholiths and treat surrounding granulation tissue (83,85,86). Cryotherapy-assisted removal of broncholiths has been successful in four cases with one patient requiring argon plasma coagulation to control minor bleeding (87-89). Patients with successful endoscopic removal tend to have small broncholiths that are not fixed in the airway, proximal location, and not contiguous with the pulmonary artery on CT scan. Thoracotomy with broncholithectomy is reserved for symptomatic mixed or extraluminal lesions that cannot be removed broncoscopically and for lesions that cause airway esophageal fistulas (86,90).
Tracheobronchopathia osteochondroplastica (TO)
TO is a benign disorder characterized by submucosal nodules containing combinations of cartilaginous, osseous, and calcified acellular protein matrix that protrudes into the bronchial lumen and spares the posterior membrane (91). Although previously identified in only 0.09% of 8,700 flexible bronchoscopies, the rate of diagnosis of TO has increased to 0.7% with increased recognition of the disease (92,93). Broncoscopically, TO appears as diffuse submucosal ivory nodules 1–10 mm in size, most frequently involving the distal 2/3 of the trachea (94). TO can rarely cause severe tracheal stenosis that makes endotracheal intubation difficult or requires treatment, such as laser therapy (95). Removal of endobronchial calcified nodules can be performed using Nd:YAG laser with or without coring with the use of the rigid bronchoscope (96).
Endobronchial hamartoma
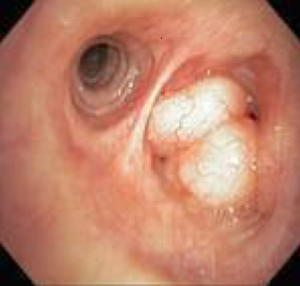
Benign tumors of the airway constitute only 2% of all lung tumors, with hamartoma being the most common (97). In the largest published series of pulmonary hamartomas, only 1.4% had an endobronchial location (98) (Figure 16). In biopsy-proven symptomatic endobronchial hamartomas, bronchoscopic intervention is the treatment of choice. Multiple procedures may be needed for complete removal due to a significant recurrence rate of 27% at 16 months. Treatment modalities include mechanical resection, laser, cryotherapy, and argon plasma coagulation (99-101). Large endobronchial hamartomas have been removed by using electrocautery snare (102-104).
CAO due to infection
A wide variety of infections including viruses, bacteria, mycobacteria, fungi, and parasites have been associated with airway obstruction. While some airway infections cause only mild mucosal changes, others may be life-threatening due to the severity of airway obstruction.
Several viral infections are known to have central airway manifestations, but viral disease compromising the patency of the central airways is rare. Herpes simplex virus (HSV), cytomegalovirus (CMV), and respiratory syncytial virus (RSV) have been reported to cause CAO with endobronchial findings including polypoid lesions and masses, mucosal irregularities, ulceration, stenosis, and plugs containing mucus and cellular debris (105-107).
Numerous infectious organisms including Corynebacterium, Staphylococcus aureus, Hemophilus, Aspergillus, CMV, HSV, and RSV have been associated with the production of pseudomembranes, a coagulum of inflammatory material and fibrin (107). Pseudomembrane formation in the tracheobronchial tree can cause respiratory failure through obstruction of the airways. Along with appropriate antibiotic therapy, re-establishing airway patency often requires bronchoscopic removal of obstructing pseudomembranes (108-109) (Figure 17).
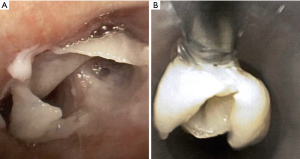
Recurrent respiratory papillomatosis (RRP)
RRP, caused by human papillomavirus types 6 and 11, results in debilitating chronic disease in both adult and pediatric patients with an estimated incidence of 2 per 100,000 adults (110). Papillomas can appear as whitish polypoid lesions with clean and smooth surfaces that arise in the tracheobronchial tree with the potential for malignant transformation to squamous cell carcinoma (111) (Figure 18). Endoscopic excision of the papilloma without damaging normal structures is the primary treatment modality, but recurrence is common (112). For removal of laryngeal papillomas, microdebriders are preferred over carbon dioxide laser, as they are associated with fewer complications (113). Tracheostomy may be required in aggressive cases with impending airway compromise and failed debulking, but decannulation should be considered as early as possible. Tracheal papillomas developed in 50% of patients with RRP who underwent tracheostomy (114).
To optimally control disease, in addition to endoscopic interventions, approximately 20% of patients with RRP require adjunctive medical treatment (113). Cidofovir, a cytosine analog, is the most commonly used and studied antiviral for RRP. Intralesional cidofovir resulted in 73.6% complete remission rates, with the majority achieving remission for more than 1 year. There was also a significant increase in the median time interval to the next procedure (115). Serial intralesional bevacizumab injections with KTP laser photoangiolysis decreased disease burden and recurrence (116,117). In a case series of five patients treated with systemic bevacizumab, all patients had a rapid and sustained response with a reduction in the number of bronchoscopic interventions required (118). It was also successfully used in a patient with multiple airway lesions and diffuse airway involvement (119). Other treatments including interferon, retinoids, zinc, and cyclooxygenase-2 inhibitors have been described in small series (120). There is insufficient evidence to determine whether photodynamic therapy alters the course of disease in patients with RRP (121).
Endobronchial tuberculosis
With an estimated 9.6 million cases of tuberculosis (TB) and an incidence of endobronchial TB ranging from 6% to 50% (122,123), endobronchial TB is by far the most common bacterial infection resulting in CAO worldwide. A preponderance of endobronchial TB has been noted in women in their second and third decades (124,125). The appearance of endobronchial TB on bronchoscopy has been classified into subtypes: actively caseating, edematous-hyperemic, fibrostenotic, tumorous, granular, ulcerative, and nonspecific bronchitic (126) (Figure 19).
Medication to treat the infection is the mainstay of therapy for endobronchial TB. Corticosteroids have not been demonstrated to prevent bronchial stenosis in adults with endobronchial TB (127). A variety of bronchoscopic therapies have been used to help re-establish airway patency including balloon dilation, hot ablative therapies, cryotherapy, topical mitomycin C, and stent placement (123). In one study, 80 patients with post-tuberculosis tracheobronchial stenosis initially underwent balloon dilation, Nd:YAG laser resection and/or bougie dilation as first-line management. Silicone stents were required in 94% to maintain airway patency, and 65% were successfully removed at a median of 14 months (128). Silicone stents placed in 71 patients with post-tuberculosis tracheobronchial stenosis were successfully removed in 40 patients at a median of 12.5 months, while stent re-insertion was necessary in 27 patients and four patients underwent surgical management (129).
Endobronchial fungal infections
Endobronchial fungal infection with manifestations ranging from mild mucosal inflammation to CAO has been described with numerous fungal species including Aspergillus, Coccidioides, Zygomycetes, Candida, Cryptococcus, and Histoplasma (130).
Aspergillus tracheobronchitis (ATB) is an uncommon presentation of invasive pulmonary aspergillosis and is associated with a high mortality rate. The population susceptible to this infection includes patients immunocompromised by a host of causes including neutropenia, hematologic malignancies, organ transplant, and long-term corticosteroid use (131). Three categories of ATB have been proposed including obstructive ATB characterized by thick mucus plugs containing Aspergillus, ulcerative ATB with focal involvement of the tracheobronchial tree, and pseudomembranous ATB caused by fungal invasion of the airway mucosa with subsequent mucosal necrosis and sloughing. Pseudomembranous tracheobronchitis is the most aggressive variant and is associated with a high fatality rate (132).
The bronchoscopic appearance of fungal tracheobronchitis varies and may include necrotizing or ulcerative mucosal lesions, pseudomembranes, white or yellow mucosal plaques, endoluminal masses and vegetation (133) (Figure 20). Bronchoscopic management of this process may be complex due to the thick, tenacious nature of the retained membranes and the risk for massive bleeding during removal of the infected tissues (134). Rigid bronchoscopic debulking, various hot thermal techniques, cryotherapy, and stenting have been used to help remove obstructing membranes and maintain airway patency (135).
Extrinsic airway compression
A variety of processes located external to the airway including diseases of the thyroid, esophagus, lymph nodes, and vascular and bony structures may cause airway obstruction through extrinsic compression. Chronic compression of the airway cartilage may also weaken the structural integrity of the airway resulting in focal malacia.
Non-malignant thyroid lesions
Benign thyroid disorders including goiters, thyroid cysts, and thyroiditis can cause obstruction of the central airways through extrinsic compression of the trachea or tracheomalacia occurring after thyroid resection (136). Substernal goiter is the most frequent cause of benign thyroid-induced airway obstruction with presenting symptoms including cough, hoarseness, and dyspnea on exertion (137).
While surgery is considered the treatment of choice for CAO due to benign thyroid disease, bronchoscopic management with stenting can be a viable therapeutic alternative. In one study, 21 stents (10 Dumon stents, 8 Tygon-Noppen stents, and 3 Ultraflex stents) were inserted in 17 patients (15 cases of large substernal goiters causing extrinsic compression of the trachea, 1 case of combined extrinsic stenosis and tracheomalacia, and 1 case of severe tracheomalacia occurring after thyroidectomy for a large substernal goiter). All patients experienced immediate symptomatic relief, as confirmed by spirometric improvement. Improvement persisted in 15 patients (88%) during a mean follow-up period of 46.4 +/− 34.4 months (range, 5 to 96 months) (138).
Fibrosing mediastinitis
Fibrosing mediastinitis is caused by the excessive proliferation of fibrous tissue in the mediastinum and may result in narrowing of mediastinal structures including the great vessels, esophagus, and trachea and bronchi, where it may cause severe stenosis. Prior infection with Histoplasma capsulatum, a fungus endemic in the central, southeast, and mid-Atlantic United States is the most common cause of this disorder (139). Methylsergide exposure, radiation, autoimmune disease and rheumatic fever have also been associated with this condition (140). In the case of histoplasmosis, leakage of fungal antigens into the mediastinum is believed to result in a hypersensitivity reaction, leading to a significant fibrotic response (139).
Presentation will depend on the location and severity of the fibrotic process. When fibrosing mediastinitis involves the tracheobronchial tree, dyspnea, cough, wheezing, hemoptysis, and post-obstructive infection may be present (141). Chest CT often shows mediastinal calcifications and narrowing of mediastinal structures (142). Biopsy is usually not needed due to the characteristic findings on CT, extensive calcification, and increased risk of bleeding secondary to engorged vessels.
No curative therapy exists for this disease and antifungal medications are usually not effective. Treatment of tracheal and bronchial stenosis due to fibrosing mediastinitis includes bronchoscopic balloon dilation, stent placement, and surgery. In a series of seven patients who underwent silicone stent placement for management of severe extrinsic airway compression, 5 were able to have the stent successfully removed (143). Risk of bleeding is increased due to concomitant vascular obstruction. Airway surgery to manage tracheobronchial stenosis is technically challenging due to intense fibrosis and is associated with a significant mortality rate (144).
Vascular causes of CAO
Tracheobronchial compression of the airway by vascular structures is more frequently seen in the pediatric population, though both congenital and acquired vascular abnormalities have been reported to cause CAO in adults. The reported cases of congenital anomalies detected in adults causing tracheobronchial compression with significant respiratory symptoms include right-sided aortic arch, double aortic arch, pulmonary artery sling, and left carotid artery anomaly. Among 36 cases of vascular tracheobronchial compression, 22 were diagnosed with right aortic arch and 10 were diagnosed with double aortic arch (145). Primary presentations in adulthood are extremely unusual and no ideal management strategy has been clarified for the symptomatic adults. Aortic aneurysms managed with endovascular repair and aortic dissection may also result in severe bronchial obstruction.
While surgical reconstruction is usually considered the treatment of choice for CAO due to compression by a vascular structure, failure of prior surgery or poor surgical candidacy may make bronchoscopic management of the stenosis the only feasible option. When considering bronchoscopic management of CAO due to extrinsic compression, careful assessment of the potential impact of the stent on the structures extrinsic to the airway is critical. Aorto-bronchial fistula formation has been described following placement of metal stents to relieve bronchial compression by a dissecting aortic aneurysm and following endoluminal stent graft repair (146,147).
Chest wall and spinal deformities causing CAO
Extrinsic compression of the tracheobronchial tree from spine and chest wall deformities including kyphoscoliosis, pectus excavatum and straight back syndrome can result in narrowing of the airway. This airway narrowing is often first noted on chest CT and confirmed with bronchoscopy. In cases of severe kyphoscoliosis, direct compression by the deviated thoracic spine, or rotation and distortion of the airway because of altered thoracic anatomy can result in bronchial compression (Figure 21).
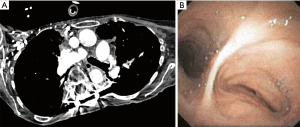
Options for management of the CAO depend on the severity and location of the narrowing and surgical candidacy of the patient. Reconstructive airway surgery has traditionally been the treatment of choice; however, minimally invasive management including balloon dilation and airway stent placement have been utilized as a bridge to surgery or in patients who fail or are not candidates for surgery (148-151).
Conclusions
There are a multitude of etiologies for benign CAO. Due to nonspecific respiratory symptoms that often mimic more common conditions, early diagnosis can be challenging. Although a variety of bronchoscopic interventions are available and highly efficacious, given the often-systemic nature and complex pathophysiology of many of these varied conditions, multidisciplinary collaboration is essential for an optimal outcome.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.07.04). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J 1999;13:888-93. [Crossref] [PubMed]
- Ko JM, Jung JI, Park SH, et al. Benign tumors of the tracheobronchial tree: CT-pathologic correlation. AJR Am J Roentgenol 2006;186:1304-13. [Crossref] [PubMed]
- Hoppe H, Walder B, Sonnenschein M, et al. Multidetector CT virtual bronchoscopy to grade tracheobronchial stenosis. AJR Am J Roentgenol 2002;178:1195-200. [Crossref] [PubMed]
- Boiselle PM, Ernst A. Recent advances in central airway imaging. Chest 2002;121:1651-60. [Crossref] [PubMed]
- Gilkeson RC, Cianocibello LM, Hejal RB, et al. Tracheobronchomalacia: dynamic airway evaluation with multi-detector CT. AJR Am J Roentgenol 2001;176:205-10. [Crossref] [PubMed]
- Lee KS, Sun MRM, Ernst A, et al. Comparison of dynamic expiratory CT with bronchoscopy for diagnosing airway malacia: a pilot evaluation. Chest 2007;131:758-64. [Crossref] [PubMed]
- Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948-68. [Crossref] [PubMed]
- Noppen M, Stratakos G, Amjadi K, et al. Stenting allows weaning and extubation in ventilator- or tracheostomy dependency secondary to benign airway disease. Respir Med 2007;101:139-45. [Crossref] [PubMed]
- Nouraei SA, Ma E, Patel A, et al. Estimating the population incidence of adult post-intubation laryngotracheal stenosis. Clin Otolaryngol 2007;32:411-2. [Crossref] [PubMed]
- Dalar L, Karasulu L, Abul Y, et al. Bronchoscopic treatment in the management of benign tracheal stenosis: choices for simple and complex tracheal stenosis. Ann Thorac Surg 2016;101:1310-7. [Crossref] [PubMed]
- Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33. [Crossref] [PubMed]
- Shin B, Kim K, Jeong BH, et al. Clinical significance of differentiating post-intubation and post-tracheostomy tracheal stenosis. Respirology 2017;22:513-20. [Crossref] [PubMed]
- Bo L, Li C, Chen M, et al. Application of electrocautery needle knife combined with balloon dilatation versus balloon dilatation in the treatment of tracheal fibrotic scar stenosis. Respiration 2018;95:182-7. [Crossref] [PubMed]
- Mehta AC, Lee FY, Cordasco EM, et al. Concentric tracheal and subglottic stenosis. Management using the Nd-YAG laser for mucosal sparing followed by gentle dilatation. Chest 1993;104:673-7. [Crossref] [PubMed]
- Fernando HC, Dekerarty D, Downie G, et al. Feasibility of cryospray and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg 2011;40:1177-80. [PubMed]
- Bhora FY, Ayud A, Forleiter CM, et al. Treatment of benign tracheal stenosis using endoluminal spray cryotherapy. JAMA Otolaryngol Head Neck Surg 2016;142:1082-7. [Crossref] [PubMed]
- Lim SY, Kim H, Jeon K, et al. Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J 2012;53:565-70. [Crossref] [PubMed]
- Martinez-Ballarin JI, Diaz-Jimenez JP, Castro MJ, et al. Silicone stents in the management of benign tracheobronchial stenoses. Tolerance and early results in 63 patients. Chest 1996;109:626-9. [Crossref] [PubMed]
- Prasanna Kumar S, Ravikumar A, Senthil K, et al. Role of Montgomery t-tube stent for laryngotracheal stenosis. Auris Nasus Larynx 2014;41:195-200. [Crossref] [PubMed]
- Colt HG, Harrell JH, Neuman TR, et al. External fixation of subglottic tracheal stents. Chest 1994;105:1653-7. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7. [Crossref] [PubMed]
- Lunn W, Garland R, Ashiku S, et al. Microdebrider bronchoscopy: a new tool for the interventional bronchoscopist. Ann Thorac Surg 2005;80:1485-8. [Crossref] [PubMed]
- Reichert LK, Zhao AS, Galati LT, et al. The efficacy of mitomycin c in the treatment of laryngotracheal stenosis: results and experiences with a difficult disease entity. ORL J Otorhinolaryngol Relat Spec 2015;77:351-8. [Crossref] [PubMed]
- Simpson CB, James JC. The efficacy of mitomycin-c in the treatment of laryngotracheal stenosis. Laryngoscope 2006;116:1923-5. [Crossref] [PubMed]
- Qiu XJ, Zhang J, Wang J, et al. Application of paclitaxel as adjuvant treatment for benign cicatricial airway stenosis. J Huazhong Univ Sci Technolog Med Sci 2016;36:817-22. [Crossref] [PubMed]
- Lee HJ, Labaki W, Yu DH, et al. Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 2017;9:4651-9. [Crossref] [PubMed]
- Wood DE, Liu YH, Vallieres E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72. [Crossref] [PubMed]
- Majid A, Kheir F, Chung J, et al. Covered balloon-expanding stents in airway stenosis. J Bronchology Interv Pulmonol 2017;24:174-7. [Crossref] [PubMed]
- Chung FT, Lin SM, Chou CL, et al. Factors leading to obstructive granulation tissue formation after Ultraflex stenting in benign tracheal narrowing. Thorac Cardiovasc Surg 2010;58:102-7. [Crossref] [PubMed]
- Chung FT, Chen HC, Chou CL, et al. An outcome analysis of self-expandable metallic stents in central airway obstruction: a cohort study. J Cardiothorac Surg 2011;6:46. [Crossref] [PubMed]
- Colt HG, Crawford SW. In vitro study of the safety limits of bronchoscopic argon plasma coagulation in the presence of airway stents. Respirology 2006;11:643-7. [Crossref] [PubMed]
- Rahman NA, Fruchter O, Shitrit D, et al. Flexible bronchoscopic management of benign tracheal stenosis: long term follow-up of 115 patients. J Cardiothorac Surg 2010;5:2. [Crossref] [PubMed]
- Allen AM, Abdelrahman N, Silvern D, et al. Endobronchial brachytherapy provides excellent long-term control of recurrent granulation tissue after tracheal stenosis. Brachytherapy 2012;11:322-6. [Crossref] [PubMed]
- Wang T, Zhang J, Wang J, et al. Paclitaxel drug-eluting tracheal stent could reduce granulation tissue formation in a canine model. Chin Med J (Engl) 2016;129:2708-13. [Crossref] [PubMed]
- Gottlieb J, Fuehner T, Dierich M, et al. Are metallic stents really safe? A long-term analysis in lung transplant recipients. Eur Respir J 2009;34:1417-22. [Crossref] [PubMed]
- Dedo HH, Catten MD. Idiopathic progressive subglottic stenosis: findings and treatment in 52 patients. Ann Otol Rhinol Laryngol 2001;110:305-11. [Crossref] [PubMed]
- Aarnæs MT, Sandvik L, Brondbo K. Idiopathic subglottic stenosis: an epidemiological single-center study. Eur Arch Otorhinolaryngol 2017;274:2225-8. [Crossref] [PubMed]
- Mark EJ, Meng F, Kradin RL, et al. Idiopathic tracheal stenosis: a clinicopathologic study of 63 cases and comparison of the pathology with chondromalacia. Am J Surg Path 2008;32:1138-43. [Crossref] [PubMed]
- Shabani S, Hoffman MR, Brand WT, et al. Endoscopic management of idiopathic subglottic stenosis. Ann Otol Rhinol Laryngol 2017;126:96-102. [Crossref] [PubMed]
- Murgu SD, Egressy K, Laxmanan B, et al. Central airway obstruction: benign strictures, tracheobronchomalacia, and malignancy-related obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Ciccone AM, De Giacomo T, Venuta F, et al. Operative and non-operative treatment of benign subglottic laryngotracheal stenosis. Eur J Cardiothorac Surg 2004;26:818-22. [Crossref] [PubMed]
- Ashiku SK, Kuzucu A, Grillo HC, et al. Idiopathic laryngotracheal stenosis: effective definitive treatment with laryngotracheal resection. J Thorac Cardiovasc Surg 2004;127:99-107. [Crossref] [PubMed]
- Ernst A, Rafeq S, Boiselle P, et al. Relapsing polychondritis and airway involvement. Chest 2009;135:1024-30. [Crossref] [PubMed]
- Lin ZQ, Xu JR, Chen JJ, et al. Pulmonary CT findings in relapsing polychondritis. Acta Radiol 2010;51:522-6. [Crossref] [PubMed]
- Lee KS, Ernst A, Trentham DE, et al. Relapsing polychondritis: prevalence of expiratory CT airway abnormalities. Radiology 2006;240:565-73. [Crossref] [PubMed]
- Lei W, Zeng H, Zeng DX, et al. (18)F-FDG PET-CT: a powerful tool for the diagnosis and treatment of relapsing polychondritis. Br J Radiol 2016;89:20150695 [Crossref] [PubMed]
- Yamashita H, Takahashi H, Kubota K, et al. Utility of fluorodeoxyglucose positron emission tomography/computed tomography for early diagnosis and evaluation of disease activity of relapsing polychondritis: a case series and literature review. Rheumatology (Oxford) 2014;53:1482-90. [Crossref] [PubMed]
- Rafeq S, Trentham D, Ernst A. Pulmonary manifestations of relapsing polychondritis. Clin Chest Med 2010;31:513-8. [Crossref] [PubMed]
- Hong G, Kim H. Clinical characteristics and treatment outcomes of patients with relapsing polychondritis with airway involvement. Clin Rheumatol 2013;32:1329-35. [Crossref] [PubMed]
- Martinez Del Pero M, Rasmussen N, Chaudhry A, et al. Structured clinical assessment of the ear, nose and throat in patients with granulomatosis with polyangiitis (Wegener’s). Eur Arch Otorhinolaryngol 2013;270:345-54. [Crossref] [PubMed]
- Terrier B, Dechartres A, Girard C, et al. Granulomatosis with polyangiitis: endoscopic management of tracheobronchial stenosis: results from a multicenter experience. Rheumatology (Oxford) 2015;54:1852-7. [Crossref] [PubMed]
- Martinez Del Pero M, Jayne D, Chaudhry A, et al. Long-term outcome of airway stenosis in granulomatosis with polyangiitis (Wegener granulomatosis): an observation study. JAMA Otolaryngol Head Neck Surg 2014;140:1038-44. [Crossref] [PubMed]
- Nouraei SA, Obholzer R, Ind PW, et al. Results of endoscopic surgery and intralesional steroid therapy for airway compromise due to tracheobronchial Wegener’s granulomatosis. Thorax 2008;63:49-52. [Crossref] [PubMed]
- Wolter NE, Ooi EH, Witterick IJ. Intralesional corticosteroid injection and dilation provides effective management of subglottic stenosis in Wegener’s granulomatosis. Laryngoscope 2010;120:2452-5. [Crossref] [PubMed]
- Hoffman GS, Thomas-Golbanov CK, Chan J, et al. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003;30:1017-21. [PubMed]
- Jordan NP, Verma H, Siddiqui A, et al. Morbidity and mortality associated with subglottic laryngotracheal stenosis in granulomatosis with polyangiitis (Wegener’s granulomatosis): a single-center experience in the United Kingdom. J Laryngol Otol 2014;128:831-7. [Crossref] [PubMed]
- Taylor SC, Clayburgh DR, Rosenbaum JT, et al. Clinical manifestations and treatment of idiopathic and Wegener granulomatosis-associated subglottic stenosis. JAMA Otolaryngol Head Neck Surg 2013;139:76-81. [Crossref] [PubMed]
- Rasmussen N. L24. Local treatments of subglottic and tracheal stenoses in granulomatosis with polyangiitis (Wegener’s). Presse Med 2013;42:571-4. [Crossref] [PubMed]
- Wester JL, Clayburgh DR, Stott WJ, et al. Airway reconstruction in Wegener’s granulatomosis-associated laryngotracheal stenosis. Laryngoscope 2011;121:2566-71. [Crossref] [PubMed]
- O’Regan A, Fenlon HM, Beamis JF Jr, et al. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine (Baltimore) 2000;79:69-79. [Crossref] [PubMed]
- Lu X, He B, Wang G, et al. Bronchoscopic diagnosis and treatment of primary tracheobronchial amyloidosis: a retrospective analysis from China. Biomed Res Int 2017;2017:3425812 [Crossref] [PubMed]
- Serrano-Fernández ML, Alvarez-Maldonado P, Aristi-Urista G, et al. Narrow-band imaging bronchoscopy in tracheobronchial amyloidosis. J Bronchology Interv Pulmonol 2014;21:267-70. [Crossref] [PubMed]
- Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc 2000;75:1148-52. [Crossref] [PubMed]
- Attwood HD, Price CG, Riddell RJ. Primary diffuse tracheobronchial amyloidosis. Thorax 1972;27:620-4. [Crossref] [PubMed]
- Ding L, Li W, Wang K, et al. Primary tracheobronchial amyloidosis in China: analysis of 64 cases and a review of literature. J Huazhong Univ Sci Technolog Med Sci 2010;30:599-603. [Crossref] [PubMed]
- Fiorelli A, Accardo M, Galluccio G, et al. Tracheobronchial amyloidosis treated by endobronchial laser resection and self expanding Y stent. Arch Bronconeumol 2013;49:303-5. [PubMed]
- Yang S, Chia SY, Chuah KL, et al. Tracheobronchial amyloidosis treated with rigid bronchoscopy and stenting. Surg Endosc 2003;17:658-9. [Crossref] [PubMed]
- Neben-Wittich MA, Foote RL, Kalra S. External beam radiation therapy for tracheobronchial amyloidosis. Chest 2007;132:262-7. [Crossref] [PubMed]
- Ren S, Ren G. External beam radiation therapy is safe and effective in treatment primary pulmonary amyloidosis. Respir Med 2012;106:1063-9. [Crossref] [PubMed]
- Truong MT, Kachnic LA, Grillone GA, et al. Long-term results of conformal radiotherapy for progressive airway amyloidosis. Int J Radiat Oncol Biol Phys 2012;83:734-9. [Crossref] [PubMed]
- Gertz MA. How to manage primary amyloidosis. Leukemia 2012;26:191-8. [Crossref] [PubMed]
- Polychronopoulos VS, Prakash UBS. Airway involvement in sarcoidosis. Chest 2009;136:1371-80. [Crossref] [PubMed]
- Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest 2005;127:472-81. [Crossref] [PubMed]
- Carlin BW, Harrell JH 2nd, Moser KM. The treatment of endobronchial stenosis using balloon catheter dilatation. Chest 1988;93:1148-51. [Crossref] [PubMed]
- Fouty BW, Pomeranz M, Thigpen TP, et al. Dilatation of bronchial stenoses due to sarcoidosis using a flexible fiberoptic bronchoscope. Chest 1994;106:677-80. [Crossref] [PubMed]
- Teo F, Anantham D, Feller-Kopman D, et al. Bronchoscopic management of sarcoidosis related bronchial stenosis with adjunctive topical mitomycin c. Ann Thorac Surg 2010;89:2005-7. [Crossref] [PubMed]
- Fruchter O, Abed El Raouf B, Rosengarten D, et al. Long-term outcome of short metallic stents for lobar airway stenosis. J Bronchology Interv Pulmonol 2017;24:211-5. [Crossref] [PubMed]
- Chapman JT, Mehta AC. Bronchoscopy in sarcoidosis: diagnostic and therapeutic interventions. Curr Opin Pulm Med 2003;9:402-7. [Crossref] [PubMed]
- Dean CM, Sataloff RT, Hawkshaw MJ, et al. Laryngeal sarcoidosis. J Voice 2002;16:283-8. [Crossref] [PubMed]
- Seo JB, Song KS, Lee JS, et al. Broncholithiasis: review of the causes with radiologic-pathologic correlation. Radiographics 2002;22:S199-213. [Crossref] [PubMed]
- Lim SY, Lee KJ, Jeon K, et al. Classification of broncholiths and clinical outcomes. Respirology 2013;18:637-42. [Crossref] [PubMed]
- McLean TR, Beall AC Jr, Jones JW. Massive hemoptysis due to broncholithiasis. Ann Thorac Surg 1991;52:1173-5. [Crossref] [PubMed]
- Olson EJ, Utz JP, Prakash UB. Therapeutic bronchoscopy in broncholithiasis. Am J Respir Crit Care Med 1999;160:766-70. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Maniscalco L. Rigid bronchoscopy and surgical resection for broncholithiasis and calcified mediastinal lymph nodes. J Thorac Cardiovasc Surg 2008;136:186-90. [Crossref] [PubMed]
- Reddy AJ, Govert JA, Sporn TA, et al. Broncholith removal using cryotherapy during flexible bronchoscopy: a case report. Chest 2007;132:1661-3. [Crossref] [PubMed]
- Lee JH, Ahn JH, Shin AY, et al. A promising treatment for broncholiths removal using cryotherapy during flexible bronchoscopy: two case reports. Tuberc Respir Dis (Seoul) 2012;73:282-7. [Crossref] [PubMed]
- Campbell SN, Lala D, Rubio E. Cryotherapy: a viable tool to remove broncholiths under flexible bronchoscopy. Biomedicine (Taipei) 2016;6:24. [Crossref] [PubMed]
- Jin YX, Jiana GN, Jiang L, et al. Diagnosis and treatment evaluation of 48 cases of broncholithiasis. Thorac Cardiovasc Surg 2016;64:450-5. [Crossref] [PubMed]
- Meyer CN, Dossing M, Broholm H. Tracheobronchopathia osteochondroplastica. Respir Med 1997;91:499-502. [Crossref] [PubMed]
- Pérez-Rodriguez E, Nunez N, Alvarado C, et al. Diagnosis of tracheopathia osteochondroplastica. Chest 1990;97:763. [Crossref] [PubMed]
- Wang N, Long F, Jiang S. Tracheobronchopathia osteochondroplastica: two case reports and review of literature. Medicine (Baltimore) 2016;95:e3396 [Crossref] [PubMed]
- Ulasli SS, Kupeli E. Tracheobronchopathia osteochondroplastica: a review of the literature. Clin Respir J 2015;9:386-91. [Crossref] [PubMed]
- Leske V, Lazor R, Coetmeur D, et al. Tracheobronchopathia osteochondroplastica: a study of 41 patients. Medicine (Baltimore) 2001;80:378-90. [Crossref] [PubMed]
- Jabbardarjani HR, Radpey B, Kharabian S, et al. Tracheobronchopathia osteochondroplastica: presentation of ten cases and review of the literature. Lung 2008;186:293-7. [Crossref] [PubMed]
- Murray J, Kielkowski D, Leiman G. The prevalence and age distribution of peripheral pulmonary hamartomas in adult males. An autopsy-based study. S Afr Med J 1991;79:247-9. [PubMed]
- Gjevre JA, Myers JL, Prakash UB. Pulmonary hamartomas. Mayo Clin Proc 1996;71:14-20. [Crossref] [PubMed]
- Abdel Hady SM, Elbastawisy SE, Hassaballa AS, et al. Is surgical resection superior to bronchoscopic resection in patients with symptomatic endobronchial hamartoma? Interact Cardiovasc Thorac Surg 2017;24:778-82. [Crossref] [PubMed]
- Kim HR, Choi KH, Jeong ET, et al. Resection of an endobronchial hamartoma by cryotherapy. Korean J Intern Med 2016;31:805-6. [Crossref] [PubMed]
- Sim JK, Choi JH, Oh JY, et al. Two cases of diagnosis and removal of endobronchial hamartoma by cryotherapy via flexible bronchoscopy. Tuberc Respir Dis (Seoul) 2014;76:141-5. [Crossref] [PubMed]
- Liu C, Wang JJ, Zhu YH, et al. Successful use of snare electrocautery via flexible fiberoptic bronchoscopy for removal of an endobronchial hamartoma causing chronic lung atelectasis and mimicking malignancy. Ther Adv Respir Dis 2017;11:435-8. [Crossref] [PubMed]
- Lococo F, Galeone C, Lasagni L, et al. Endobronchial hamartoma subtotally occluding the right main bronchus and mimicking bronchial carcinoid tumor. Medicine (Baltimore) 2016;95:e3369 [Crossref] [PubMed]
- Kim JH, Jeong GM, Park KH, et al. Large endobronchial hamartoma successfully resected by snare through flexible bronchoscopy. Ann Thorac Surg 2015;100:1107-9. [Crossref] [PubMed]
- Naber JM, Palmer SM, Howell DN. Cytomegalovirus infection presenting as bronchial polyps in lung transplant recipients. J Heart Lung Transplant 2005;24:2109-13. [Crossref] [PubMed]
- St John RC, Pacht ER. Tracheal stenosis and failure to wean from mechanical ventilation due to herpetic tracheitis. Chest 1990;98:1520-2. [Crossref] [PubMed]
- Cortjens B, de Jong R, Bonsing JG, et al. Local dornase alpha treatment reduces NETs-induced airway obstruction during severe RSV infection. Thorax 2018;73:578-80. [Crossref] [PubMed]
- Imoto EM, Stein RM, Shellito JE, et al. Central airway obstruction due to cytomegalovirus-induced necrotizing tracheitis in a patient with AIDS. Am Rev Respir Dis 1990;142:884-6. [Crossref] [PubMed]
- Pervez NK, Kleinerman J, Kattan M, et al. Pseudomembrane necrotizing bronchial aspergillosis. A variant of invasive aspergillosis in a patient with hemophilia and acquired immunodeficiency syndrome. Am Rev Respir Dis 1985;131:961-3. [PubMed]
- Carifi M, Napolitano D, Morandi M, et al. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag 2015;11:731-8. [Crossref] [PubMed]
- Fortes HR, von Ranke FM, Escuissato DL, et al. Recurrent respiratory papillomatosis: a state-of-the-art review. Respir Med 2017;126:116-21. [Crossref] [PubMed]
- Wilcox LJ, Hull BP, Baldassari CM, et al. Diagnosis and management of recurrent respiratory papillomatosis. Pediatr Infect Dis J 2014;33:1283-4. [Crossref] [PubMed]
- Schraff S, Derkay CS, Burke B, et al. American society of pediatric otolaryngology members’ experience with recurrent respiratory papillomatosis and the use of adjuvant therapy. Arch Otolaryngol Head Neck Surg 2004;130:1039-42. [Crossref] [PubMed]
- Cole RR, Myer CM 3rd, Cotton RT. Tracheotomy in children with recurrent respiratory papillomatosis. Head Neck 1989;11:226-30. [Crossref] [PubMed]
- Drejet S, Halum S, Brigger M, et al. A systematic review: outcomes in adult recurrent respiratory papillomatosis treated with Intralesional cidofovir or bevacizumab. Otolaryngol Head Neck Surg 2017;156:435-41. [Crossref] [PubMed]
- Zeitels SM, Lopez-Guerra G, Burns JA, et al. Microlaryngoscopic and office-based injection of bevacizumab (Avastin) to enhance 532-nm pulsed KTP laser treatment of glottal papillomatosis. Ann Otol Rhinol Laryngol Suppl 2009;201:1-13. [PubMed]
- Zeitels SM, Barbu AM, Landau-Zemer T, et al. Local injection of bevacizumab (Avastin) and angiolytic KTP laser treatment of recurrent respiratory papillomatosis of the vocal folds: a prospective study. Ann Otol Rhinol Laryngol 2011;120:627-34. [Crossref] [PubMed]
- Mohr M, Schliemann C, Biermann C, et al. Rapid response to systemic bevacizumab therapy in recurrent respiratory papillomatosis. Oncol Lett 2014;8:1912-8. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Vial MR, et al. Recurrent respiratory papillomatosis and bevacizumab treatment. Am J Respir Crit Care Med 2018;197:539-41. [Crossref] [PubMed]
- Gallagher TQ, Derkay CS. Pharmacotherapy of recurrent respiratory papillomatosis: an expert opinion. Expert Opin Pharmacother 2009;10:645-55. [Crossref] [PubMed]
- Lieder A, Khan MK, Lippert BM. Photodynamic therapy for recurrent respiratory papillomatosis. Cochrane Database Syst Rev 2014;CD009810 [PubMed]
- Siow WT, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis 2017;9:E71-7. [Crossref] [PubMed]
- Faisal M, Harun H, Hassan TM, et al. Treatment of multiple-level tracheobronchial stenosis secondary to endobronchial tuberculosis using bronchoscopic balloon dilatation with topical mitomycin-C. BMC Pulm Med 2016;16:53. [Crossref] [PubMed]
- Lee JH, Park SS, Lee DH, et al. Endobronchial tuberculosis. Clinical and bronchoscopic features in 121 cases. Chest 1992;102:990-4. [Crossref] [PubMed]
- Jung SS, Park HS, Kim JO, et al. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology 2015;20:488-95. [Crossref] [PubMed]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [Crossref] [PubMed]
- Park IW, Choi BW, Hue SH. Prospective study of corticosteroid as an adjunct in the treatment of endobronchial tuberculosis in adults. Respirology 1997;2:275-81. [Crossref] [PubMed]
- Ryu YJ, Kim H, Yu CM, et al. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur Respir J 2006;28:1029-35. [Crossref] [PubMed]
- Lim SY, Park HK, Jeon K, et al. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011;16:959-64. [Crossref] [PubMed]
- Karnak D, Avery RK, Gildea TR, et al. Endobronchial fungal disease: an under-recognized entity. Respiration 2007;74:88-104. [Crossref] [PubMed]
- Patterson TF, Kirkpatrick WR, White M, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79:250-60. [Crossref] [PubMed]
- Denning DW. Invasive aspergillosis. Clin Infect Dis 1998;26:781-803. [Crossref] [PubMed]
- Keshishyan S, DeLorenzo L, Hammoud K, et al. Infections causing central airway obstruction: role of bronchoscopy in diagnosis and management. J Thorac Dis 2017;9:1707-24. [Crossref] [PubMed]
- Putnam JB Jr, Dignani C, Mehra RC, et al. Acute airway obstruction and necrotizing tracheobronchitis from invasive mycosis. Chest 1994;106:1265-7. [Crossref] [PubMed]
- Dhillon SS, Saoud M, Harris K. Complex multimodality central airway management of aspergillus pseudomembranous tracheobronchitis. J Thorac Dis 2017;9:915-9. [Crossref] [PubMed]
- Alfonso A, Christoudias G, Amaruddin Q, et al. Tracheal or esophageal compression due to benign thyroid disease. Am J Surg 1981;142:350-4. [Crossref] [PubMed]
- Anders HJ. Compression syndromes caused by substernal goiters. Postgrad Med J 1998;74:327-9. [Crossref] [PubMed]
- Noppen M, Poppe K, D'Haese J, et al. Interventional bronchoscopy for treatment of tracheal obstruction secondary to benign or malignant thyroid disease. Chest 2004;125:723-30. [Crossref] [PubMed]
- Goodwin RA. NIckell JA, Des Prez RM. Mediastinal fibrosis complicating healed primary histoplasmosis and tuberculosis. Medicine (Baltimore) 1972;51:227. [Crossref] [PubMed]
- Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. Radiographics 2001;21:737-57. [Crossref] [PubMed]
- Loyd JE, Tillman BF, Atkinson JB, et al. Mediastinal fibrosis complicating histoplasmosis. Medicine (Baltimore) 1988;67:295-310. [Crossref] [PubMed]
- Weinstein JB, Aronberg DJ, Sagel SS. CT of fibrosing mediastinitis: findings and their utility. AJR Am J Roentgenol 1983;141:247-51. [Crossref] [PubMed]
- Kern R, Peikert T, Edell E, et al. Bronchoscopic management of airway compression due to fibrosing mediastinitis. Ann Am Thorac Soc 2017;14:1353-5. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC. Clinical manifestation of mediastinal fibrosis and histoplasmosis. Ann Thorac Surg 1992;54:1053-7. [Crossref] [PubMed]
- Kanabuchi K, Noguchi N, Kondo T. Vascular tracheobronchial compression syndrome in adults: a review. Tokai J Exp Clin Med 2011;36:106-11. [PubMed]
- Katayama Y, Suzuki H, Mizutani T. Aorto-bronchial fistula after implantation of a self-expanding bronchial stent in a patient with aortic dissection. Jpn J Thorac Cardiovasc Surg 2000;48:73-5. [Crossref] [PubMed]
- Abdul-Ghani A, Pisipati S, McWilliams R, et al. Aorto-bronchial fistula following aortic and bronchial stenting of a thoracic aneurysm. Eur J Cardiothorac Surg 2006;29:419-21. [Crossref] [PubMed]
- Grillo HC, Wright CD, Dartevelle PG, et al. Tracheal compression caused by straight back syndrome, chest wall deformity, and anterior spinal displacement: techniques for relief. Ann Thorac Surg 2005;80:2057-62. [Crossref] [PubMed]
- Donnelly LF, Bisset GS 3rd. Airway compression in children with abnormal thoracic configuration. Radiology 1998;206:323-6. [Crossref] [PubMed]
- Sriratanaviriyakul N, Nguyen LP, Ismail H, et al. Temporary endobronchial stent as a bridge to corrective surgery for severe kyphoscoliosis-associated central-airway extrinsic compression. J Bronchology Interv Pulmonol 2016;23:331-5. [Crossref] [PubMed]
- Al-Kattan K, Simonds A, Chung KF, et al. Kyphoscoliosis and bronchial torsion. Chest 1997;111:1134-7. [Crossref] [PubMed]
Cite this article as: Holden VK, Channick CL. Management of benign central airway obstruction. AME Med J 2018;3:76.