
Primary spontaneous pneumothorax: a pathway to practice
Introduction
Primary spontaneous pneumothorax (PSP) is a pneumothorax that occurs without an iatrogenic, traumatic, or underlying lung disease related cause (1). This process typically presents in young, thin, males with no underlying lung disease. In contrast, a secondary spontaneous pneumothorax often occurs in older patients with underlying lung disease, trauma patients, and patients with an iatrogenic mechanism. The normal anatomy and physiology of the intrathoracic cavity consists of a space between parietal pleura along the chest wall and the visceral pleura along the lung parenchyma. When the diaphragm and accessory muscles contract, the intrathoracic cavity increases in volume creating negative pressure allowing air to fill the lungs. On exhalation, the muscles relax and the lungs decrease in volume, exhaling the inspiratory volume.
In the event of a pneumothorax, normal physiology is altered. During inhalation, the negative intrathoracic pressure will pull air out of the lung through the defect in the visceral pleura and into the chest cavity. Air will continue to fill this space between the parietal and visceral pleurae until the opening pressure of the defect is matched by the accumulated pressure of air in the space. At this point the pneumothorax will cease to accumulate and equilibrium will be reached. If the defect seals, then the lung will slowly re-expand as the air in the space gets absorbed. If the defect in the visceral pleura is large enough, air will continue to accumulate in the chest cavity (between parietal and visceral pleurae), further collapsing the lung, shifting the mediastinum to the contralateral side, and decreasing venous return to the right atrium. This is the physiologic basis of the clinical entity of tension pneumothorax. Given the lack of significant underlying lung pathology such as is seen in emphysema, cavitary infections, cystic fibrosis, or cavitating lung cancers, tension pneumothorax is a rare entity in PSP. In PSP, there are typically small blebs seen at the apices of the lungs present on CT imaging but often overlooked. In some cases, the disease is largely sub pleural with minimal to no abnormalities seen on CT imaging but nonetheless a defect in the visceral pleura is sustained.
Spontaneous pneumothorax has long been believed to occur most often in tall, thin, Caucasian males and often occurs at rest, with no precipitating etiology (1,2). Age-adjusted incidence in males is 7.4–18.0 cases per 100,000, and 1.2–6.0 cases per 100,000 in females (3,4). Among medical and surgical practitioners, wide variance in both initial and recurrent management techniques of patients with PSP exists, and has been a source of debate. Management strategies are highly variable throughout both pediatric and adult disciplines that treat this disorder (Table 1). Current diagnostic and treatment modalities can range from observation to tube placement to operative intervention (Table 2).
Table 1
| Family practice/pediatrics/internal medicine |
| Adult/pediatric emergency medicine |
| Interventional radiology |
| Adult/pediatric pulmonology |
| Trauma surgery |
| Adult/pediatric general surgery |
| Adult/pediatric cardiac surgery |
| General thoracic surgery |
Table 2
| Modality | Less invasive | Moderately invasive | Most invasive |
|---|---|---|---|
| A. Diagnostic modalities | |||
| Patient assessments | Clinical exam: history & physical exam | CXR* | CT scan |
| Observation location | Outpatient | Inpatient | Inpatient with oxygen |
| B. Treatment modalities—pleural space management | |||
| Decompression | Needle aspiration | Pigtail | Large bore chest tube+ |
| Tube-directed chemical pleurodesis | Blood patch | Hypertonic saline, minocycline, doxycycline, bleomycin, tetracycline | Talc, silver nitrate, fibrin glue |
| C. Treatment modalities—surgical intervention | |||
| Approaches | None | Minimally invasive surgery: VATS or robotics | Thoracostomy+ |
| Lung management | No resection | Wedge resection of bullous changes | – |
| Pleurodesis: chemical | None | Blood, hypertonic saline, minocycline | Talc, silver nitrate, fibrin glue |
| Pleurodesis: mechanical | None | Abrasion | Pleurectomy |
| Timing of surgery | 3rd occurrence or later | 2nd occurrence | 1st occurrence |
*, necessary in most cases; +, not necessary in most cases; CXR, chest X-ray; CT scan, computed tomography; VATS, video-assisted thoracoscopic surgery.
Treatment practices are inconsistent furthermore; clinicians often do not consider surgical intervention until the second (or consecutive) recurrence. For patients with recurrent disease, a surgical correction may be warranted to stop the cycle of recurrence. Greater than 90% of PSPs are treated in the hospital (5). As such, this disease process is associated with a great financial cost to patients and the healthcare system. In addition to the financial burden, there are hours of lost productivity, decreased quality of life due to periods of shortness of breath, chest pain, and discomfort, and strain on patient support networks (5,6).
Etiology
As many as 90% of patients with PSP have blebs or bullae present at the time of occurrence (7). This has led many clinicians to believe that bleb rupture is the cause of PSP. However, this may not be entirely true. Often at time of surgery, other lesions may be identified such as visceral pleura with increased porosity and areas of disrupted mesothelial cells, allowing air to leak into the pleural space (1,8,9). Recurrence rates post-bullectomy without pleurodesis are as high as 20% (8). Recurrence rates are potentially due to noxious stimuli from associated factors. These factors may include the following: tobacco use, anatomic variants and low body mass index (10-12). Case reports indicate potential familial PSP which follow an autosomal dominant inheritance pattern (13). Further study of this relationship is warranted to identify the specific pathophysiology associated with PSP pathophysiology.
The causes of secondary spontaneous pneumothorax include: airway disorders such as emphysema, congenital anomalies, infectious lung disease, interstitial lung disease, connective tissue disorders, and malignant conditions (Table 3) (1). Unlike a PSP, a secondary pneumothorax is often larger and takes much longer to resolve. This is due to the underlying lung disease that impedes the body’s ability to heal the alveolar-pleural defect.
Table 3
| Airway diseases |
| Chronic obstructive pulmonary disease |
| Severe asthma |
| Cystic fibrosis |
| Alpha-1 antitrypsin deficiency |
| Congenital bullous emphysema |
| Infectious lung diseases |
| Tuberculosis |
| Necrotizing pneumonia |
| Pneumocystis Carni pneumonia |
| Interstitial lung diseases |
| Sarcoidosis |
| Histiocytosis X |
| Catamenial |
| Idiopathic pulmonary fibrosis |
| Lymphangioleiomyomatosis |
| Connective tissue diseases |
| Marfan’s syndrome |
| Ehler-Danlos disease |
| Malignant diseases |
| Lung cancer |
| Sarcoma |
Rationale for intervention
To understand appropriate management of PSP, goals of management must first be established. The goals of management focus on alleviation of symptoms, prevention of tension physiology, avoidance of trapped lung, and reduction in risk of recurrence. Symptoms can include pleuritic chest pain, cough and shortness of breath. As the visceral and parietal pleura separate, pain receptors are activated. With every inspiration effort these receptors are stretched leading to pleuritic chest pain. The loss of functional lung volume due to external compression leads to shortness of breath. Patients will often cough as the body attempts to reopen the collapsed lung and expel the mucus from atelectatic portions of the lung. When symptoms are severe, placement of chest tube with evacuation of air in the pleural space can relieve symptoms.
It is rare for a PSP to cause tension physiology (hypotension secondary to decreased cardiac preload) given the small visceral pleural defect (8). However, this occurs more frequently in a secondary spontaneous pneumothorax. Presence of tension physiology or large lung collapse with mediastinal shift on chest X-ray (CXR) should lead to urgent need for chest decompression and chest tube placement. An additional feared complication of pneumothorax is a trapped lung. A trapped lung is caused by a fibrinous visceral pleural peel (14). As a pneumothorax forms the parietal and visceral pleura are separated causing a local inflammatory reaction. This is manifest as a fibrinous rind covering the visceral pleura. If the lung is not fully expanded while this rind is formed, the rind can serve as a restrictive capsule preventing future re-expansion of the lung. This is the basis for an early re-expansion approach to pneumothorax management.
Evaluation—diagnostic and treatment modalities
Diagnostic modalities
Table 2 demonstrates the various forms of PSP treatment modalities, stratified by level of invasiveness. Figure 1 is a management algorithm followed by the authors at a single, high-volume institution that treats PSP. Diagnostic and treatment plans for PSP must be tailored to the size of pneumothorax.
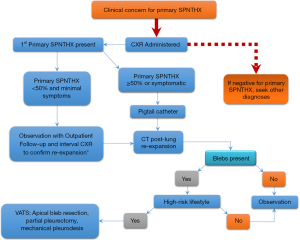
Patients with PSP commonly present to a hospital emergency department or an outpatient clinic. Clinical exams will be part of an initial evaluation. The clinical exam begins by a comprehensive medical and social history plus a physical exam. History will focus on risk factors such as smoking history, underlying lung disease [chronic obstructive pulmonary disease (COPD), asthma, lung cancer, etc.], recent illness, prior pneumothoraces, recent trauma, and history of connective tissue disorders. Questions concerning a patient’s lifestyle such as travel habits and vocation that involve the drastic fluctuation in altitude, air pressure, or water pressure should be noted. Patients, who spend extended periods of time in areas with inadequate or unavailable healthcare, usually warrant more aggressive management (example: people who live in rural geographic locations). Physical exam should include auscultation of the chest, assessing chest percussion, and tactile fremitus.
When concern exists for possible pneumothorax, a CXR should be obtained in an upright position to allow a pneumothorax to present at the lung apex. This is to determine the location and size of the potential PSP. If a significant pneumothorax is present on CXR (commonly defined as 50% of hemithorax volume), chest tube decompression with a pigtail catheter may be performed. After chest tube decompression, a computed tomography (CT) scan can be obtained to evaluate for underlying lung disease, blebs, and bullae presence. Size of pneumothorax on radiographic imaging does not correlate well with clinical manifestations (15). A pneumothorax is commonly categorized as large versus small based on if the edge of lung parenchyma is greater than 2–3 cm from chest wall on imaging. Greater than 2 cm from chest wall is chosen by the British Thoracic Society, as it has been shown to correlate with pneumothorax greater than 50% of chest capacity (16). This is in contrast to the consensus guidelines by the American College of Chest Physicians who defined large PSP as those greater than 3 cm from the chest wall (17).
A small pneumothorax defined as ≤50% of hemithorax volume have been successfully managed with observation alone (18,19). Successful management of patients with small PSPs using observation alone has been demonstrated in upwards of 80% of cases (19). Patients with small PSPs have potentially lower recurrence rates when observed, compared to after chest drainage procedure (19). When observation is elected, the patient should be provided with 100% oxygenation, as this has been demonstrated to increase reabsorption time of pneumothorax by as much as four times (20). The resolution rate of a pneumothorax has been estimated at 1.3–2.2% of the volume of the hemithorax per 24 hours (17,21-24). Repeat CXR is often obtained 4–6 hours after diagnosis to ensure the pneumothorax has not enlarged. Once pneumothorax has been demonstrated to be stable (not enlarging), the patient may be discharged to home with close outpatient follow-up.
Treatment modalities—pleural space management
Decompression—needle aspiration
Needle aspiration can be used to decompress the thoracic cavity when the patient is symptomatic (pleuric pain, short of breath, and hypoxic). Studies evaluating needle aspiration versus large bore chest tube drainage demonstrated no significant difference in successful resolution of pneumothorax (25,26). Since the advent of smaller pigtail chest tube drainage, a randomized control study demonstrated no significant difference between needle aspiration and pigtail chest tube drainage in initial success and 1- and 2-year recurrence rates for the treatment of PSP (27). The study did demonstrate a shorter length of stay in the needle aspiration group. Despite these studies, many clinicians find small bore pigtail chest tube catheters simpler to place and manage. Success rates of needle aspiration are estimated at 51–69% (28,29). This means one third, to half of patients will require a second procedure. When needle aspiration is unsuccessful, a second aspiration should not be attempted (16). In this case, a small bore chest tube should be inserted.
Decompression—tube thoracotomy
Many studies demonstrated that small bore (<14 French) Seldinger chest tubes have a similar efficacy and less pain to large bore tubes for the management of pneumothorax (30-32). While the American College of Chest Physicians consensus paper recommends use of large bore chest tubes (24–28 French) when there is concern for large air leaks on positive pressure ventilation, the British Thoracic Society guidelines recommends against use of large bore chest tubes (16,17). Placing the chest tube to suction can cause the parenchymal defect to remain open as air is continuously suctioned from the airway into the pleural cavity. When a pneumothorax is present for multiple days prior to drainage, placing the chest tube to suction can lead to rapid re-expansion of the lung and re-expansion pulmonary edema (33). Both American and British guidelines recommend use of suction if there is incomplete lung expansion with initial chest tube to water seal (16,17).
Tube-directed chemical pleurodesis
Management of spontaneous pneumothorax also attempts to address potential for recurrence. Surgical and chemical pleurodesis are methods that cause an inflammatory reaction between parietal and visceral pleura to produce symphysis between the two layers. There is no current standard, although surgical intervention with chemical or mechanical pleurodesis is routinely used in many centers. Due to lower success rates, tube-directed chemical pleurodesis is often reserved for patients who are poor surgical candidates or are unwilling to undergo surgery (16,34). Involvement of a thoracic surgeon to evaluate a patients eligibility for surgery prior to tube directed chemical pleurodesis is advised.
Originally chemical pleurodesis was done with tetracycline, however the decreased availability of this drug has led to the use of other agents such as 3.0% sodium chloride (NaCl), doxycycline, minocycline, and bleomycin. Chemical pleurodesis has been shown to decrease pneumothorax recurrence rates. One study evaluating aspiration and drainage alone compared to aspiration and drainage plus minocycline pleurodesis demonstrated a decreased recurrence rate at 12 months from 49.1% to 29.2% (35). Additional studies have demonstrated reduction in recurrence rate from 35–41% with tube thoracostomy alone to 0–29%, with addition of blood patch pleurodesis (36,37). These rates are still higher than a 5–15% recurrence rate observed after surgical intervention (38-41).
Tetrocycline and minocycline have demonstrated effectiveness at creating pleural fibrosis with low complication profiles. Minocycline has been shown to cause chest pain upon installation however this pain usually resolves (34). Additional studies have demonstrated that minocycline is effective at preventing recurrence of pneumothorax without causing chronic pain or decreasing pulmonary function (42,43).
Bleomycin has been suggested for chemical pleurodesis in malignant effusions. One study in rabbit models found that bleomycin is not effective in stimulating pleural fibrosis (44). Bleomycin’s lack of effectiveness combined with the relatively high cost has led to the limited clinical use of this drug. Given these issues, the complexities of handling the drug, and cost—it has no role in the management of PSP.
One area of controversy is the use of talc pleurodesis. At least 32 cases of acute respiratory distress syndrome (ARDS) after talc pleurodesis have been reported (34). Concern has also been raised for an increased incidence of empyema and restrictive lung disease with talc (34). Some clinicians have argued these findings are dependent on the size and dose of talc particles. One study of 418 patients diagnosed with spontaneous pneumothorax demonstrated no incidence of ARDS or pneumonitis after low doses of talc (2 g), where medium-sized talc particles (31.5 µm) were utilized (45). Despite this, due to the concern for ARDS, talc pleurodesis is not a recommended form of chemical pleurodesis. In addition, talc may has been associated with chronic pain and increased incidence of future malignancy. Future operative intervention post-talc, silver nitrate, or fibrin glue pleurodesis can be problematic due to the intense foreign body reaction and subsequent adhesions formed.
Treatment modalities—surgical intervention
The goal of surgical intervention for a PSP is to remove the diseased portion of the lung that caused the pneumothorax as well as facilitate symphysis between the parietal and visceral pleura. To accomplish this, surgeons perform bleb resections as well as pleurodesis.
Most patients with a PSP will have bullous disease identifiable on preoperative CT imaging. For patients with PSP, bullae are often isolated to focal areas most commonly the apex. Minimally invasive surgical techniques such as robotics and video-assisted thoracoscopic surgery (VATS) have been shown to be a safe and effective surgical approach to resection of bullous disease (38,39). In most cases, an open thoracotomy is not warranted. The area of bullous disease is removed with wedge resection. The use of wedge resection for treatment of spontaneous pneumothorax has been shown to be effective with a long-term recurrence rate of 5–15% (38-41).
Much controversy exists in the literature if pleurodesis at the time of surgery is beneficial. Some studies have demonstrated similar recurrence rates between patients undergoing wedge resection alone versus those undergoing wedge resection and pleurodesis (40,41). These studies are limited by small sample sizes and short follow-up. To provide clarity to this question, a 2017 meta-analysis of 51 studies including nearly 7,000 patients evaluated recurrence rates after pleurodesis (46). This study found that wedge resection with chemical pleurodesis and wedge resection as well as chemical and mechanical pleurodesis, had the lowest recurrence rates at 1.7% and 2.8%, respectively (46). Due to improved results with pleurodesis, the authors of this paper routinely perform pleurodesis at the time of wedge resection.
Pleurodesis at time of surgery can be performed either mechanically or chemically. Mechanical pleurodesis can be accomplished by either performing a pleurectomy or mechanically irritating the pleura. This can be accomplished either by direct mechanical abrasion or scoring tissue with electrocautery. Chemical pleurodesis can be accomplished with any of the chemicals mentioned above. The advantages and disadvantages of each chemical are the same as when instilled through a chest tube.
Treatment modalities—timing and management of PSP
1st occurrence
To develop an appropriate management strategy for this patient population, many factors must be considered. Anytime a pneumothorax is suspected based on history and physical exam, a chest radiograph should be obtained. If a pneumothorax is identified, establish if it is a PSP verses due to secondary causes. If the PSP is <50% and the patient is asymptomatic then observation is warranted. Observation can be conducted in the clinic or in the hospital depending on social factors. If the pneumothorax is ≥50% or patient is symptomatic, a pigtail catheter should be inserted. For all PSP patients once the lung is re-expanded, a chest CT scan should be obtained to evaluate bullous disease. Most patients do not warrant surgical intervention after a first time spontaneous pneumothorax. However, surgical intervention may be warranted if there is significant focal bullous disease seen on CT scan or if patient’s lifestyle places them away from access to healthcare for prolonged or critical periods of time such as airplane pilots, scuba divers, or frequent international travel.
If significant bullous disease is observed, patient is without access to healthcare for prolonged and/or critical periods of time, have recurrent disease, a thoracic surgeon should be consulted. If there is no thoracic surgeon at the institution of treatment, discussion with a thoracic surgeon at a surrounding facility should be sought. If surgical intervention is indicated, the patient should be transferred to a facility with thoracic surgical capabilities. If surgical intervention is determined to be appropriate VATS with wedge resection, and pleurodesis are the surgical treatments of choice. All pneumothorax patients who use tobacco should receive smoking cessation counseling prior to discharge as well as at follow-up every clinic appointment.
Recurrence
Risk factors for recurrence include female gender, above average heights for males, and lack of smoking cessation (47). Patients who ever have a recurrence are at a higher risk of future recurrences, as are those with severe bleb disease. Strenuous physical activity is not associated with recurrence risk, no activity restrictions should be placed on a patient with a history of pneumothorax (1).
When a patient presents with a concerning history and physical exam for a possible recurrent pneumothorax, a chest radiograph should be obtained. Decision to place a pigtail catheter is made based on the same algorithm as used for the first occurrence. If no prior CT scan has been performed, a CT scan should be obtained upon lung re-expansion to evaluate for bullous disease. Due to a higher recurrence rate after subsequent pneumothorax events, clinicians treating a patient with a recurrent pneumothorax should have a low threshold for operative intervention. If the algorithm was followed during the first PSP and CT scan was obtained, we will often bring patients directly to the operating room upon recurrence instead of intervening with a pigtail. Fortunately for patients, tension physiology rarely occurs with a spontaneous pneumothorax (48). Due to this, the decision for operative intervention is based on risk tolerance and convenience. Both patient and care giver’s level of education about the disease process, access to health care and providers, and social support system all play a role in development of a comprehensive management plan.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.11.05). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. Dr. Mahajan served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from Aug 2017 to Dec 2020. Dr. Khandhar served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Noppen M. Spontaneous pneumothorax: Epidemiology, pathophysiology, and cause. Eur Respir Rev 2010;19:217-19. [Crossref] [PubMed]
- Gayatridevi Y, Usharani N, Premkumar A, et al. Clinical profile of spontaneous pneu-mothorax in adults: A retrospective study. Indian J Chest Dis Allied Sci 2015;57:219-23. [PubMed]
- Bense L, Eklund G, Wilman LG. Smoking and the increased risk of contracting sponta-neous pneumothorax. Chest 1987;92:1009-12. [Crossref] [PubMed]
- Melton LJ III, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Bense L, Wiman LG, Jendteg S, et al. Economic costs of spontaneous pneumothorax. Chest 1991;99:260-1. [Crossref] [PubMed]
- Roeggla M, Roeggla G, Muellner M, et al. The cost of treatment of spontaneous pneu-mothorax with the thoracic vent compared with conventional thoracic drainage. Chest 1996;110:303. [Crossref] [PubMed]
- Donahue DM, Wright CD, Viale G, et al. Resection of pulmonary blebs and pleurodesis for spontaneous pneumothorax. Chest 1993;104:1767-9. [Crossref] [PubMed]
- Matthys H. Spontaneous pneumothorax. Multidiscip Respir Med 2011;6:6-7. [Crossref] [PubMed]
- Masshoff W, Höfer W. Zur pathologie des sogenannten idiopathischen spontanpneu-mothorax. Dtsch Med Wochenschr 1973;98:801-5. [Crossref] [PubMed]
- Bense L, Lewander R, Eklund G, et al. Nonsmoking, non-alpha 1-antitrypsin deficien-cy-induced emphysema in nonsmokers with healed spontaneous pneumothorax, identified by computed tomography of the lungs. Chest 1993;103:433-8. [Crossref] [PubMed]
- Withers JN, Fishback ME, Kiehl PV, et al. Spontaneous pneumothorax. Suggested etiology and comparison of treatment methods. Am J Surg 1964;108:772-6. [Crossref] [PubMed]
- Coxson HO, Chan IH, Mayo JR, et al. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004;170:748-52. [Crossref] [PubMed]
- Morrison PJ, Lowry RC, Nevin NC. Familial primary spontaneous pneumothorax con-sistent with true autosomal dominat inheritance. Thorax 1998;53:151-2. [Crossref] [PubMed]
- Huggins J, Doelken P, Sahn S. The unexpandable lung. F1000 Med Resp 2010;2:77.
- Vail WJ, Alway AE, England NJ. Spontaneous pneumothorax. Dis Chest 1960;38:512-15. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax. An American College of Chest Physicians Delphi Consensus Statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Stradling P, Poole G. Conservative management of spontaneous pneumothorax. Thorax 1966;21:145-9. [Crossref] [PubMed]
- O'Rourke JP, Yee ES. Civilian spontaneous pneumothorax: Treatment options and long term results. Chest 1989;96:1302-6. [Crossref] [PubMed]
- Northfield TC. Oxygen therapy for spontaneous pneumothorax. BMJ 1971;4:86-8. [Crossref] [PubMed]
- Kircher LT Jr, Swartzel RL. Spontaneous pneumothorax and its treatment. J Am Med Assoc 1954;155:24-9. [Crossref] [PubMed]
- Kelly AM, Druda D. Comparison of size classification of primary spontaneous pneu-mothorax by three international guidelines: A case for international consensus? Respir Med 2008;102:1830-2. [Crossref] [PubMed]
- Hoi K, Turchin B, Kelly AM. How accurate is the light index for estimating pneumothorax size? Australas Radiol 2007;51:196-8. [Crossref] [PubMed]
- Kelly AM, Loy J, Tsang AY, et al. Estimating the rate of re-expansion of spontaneous pneumothorax by a formula derived from computed tomography volumetry studies. Emerg Med J 2006;23:780-2. [Crossref] [PubMed]
- Noppen M, Alexander P, Driesen P, Slabbynck H, Verstraeten A. Manual aspiration versus chest tube drainage in first episodes of primary spontaneous pneumothorax. Am J Respir Crit Care Med 2002;165:1240-4. [Crossref] [PubMed]
- Harvey J, Prescott RJ. Simple aspiration versus intercostal tube drainage for spontaneous pneumothorax in patients with normal lungs. BMJ 1994;309:1338-9. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Aspiration versus tube drainage in primary spontaneous pneumothorax: A randomised study. Eur Respir J 2006;27:477-82. [Crossref] [PubMed]
- Camuset J, Laganier J, Brugiere O, et al. Needle aspiration as first-line management of primary spontaneous pneumothorax. Presse Med 2006;35:765-8. [Crossref] [PubMed]
- Chan SS, Lam PK. Simple aspiration as initial treatment for primary spontaneous pneumothorax: results of 91 consecutive cases. J Emerg Med 2005;28:133-8. [Crossref] [PubMed]
- Vedam H, Barnes DJ. Comparison of large- and small-bore intercostal catheters in the management of spontaneous pneumothorax. Intern Med J 2003;33:495-9. [Crossref] [PubMed]
- Clementsen P, Evald T, Grode G, et al. Treatment of malignant pleural effusion: Pleu-rodesis using a small percutaneous catheter. A prospective randomized study. Respir Med 1998;92:593-6. [Crossref] [PubMed]
- Akowuah E, Ho EC, George R, et al. Less pain with flexible fluted silicone chest drains than with conventional rigid chest tubes after cardiac surgery. J Thorac Cardiovasc Surg 2002;124:1027-8. [Crossref] [PubMed]
- Matsuura Y, Nomimura T, Nurikami H, et al. Clinical analysis of re-expansion pulmonary edema. Chest 1991;100:1562-6. [Crossref] [PubMed]
- How CH, Hsu H, Chen J. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc 2013;112:749-55. [Crossref] [PubMed]
- Chen JS, Chan W, Tsai K, et al. Simple aspiration and drainage and intrapleural minocy-cline pleurodesis versus simple aspiration and drainage for the initial treatment of pri-mary spontaneous pneumothorax: An open-label, parallel-group, prospective, ran-domised, controlled trial. Lancet 2013;381:1277-82. [Crossref] [PubMed]
- Manley K, Coonar A, Wells F, et al. Blood patch for persistent air leak: A review of the current literature. Curr Opin Pulm Med 2012;18:333-8. [Crossref] [PubMed]
- Lang-Lazdunski L, Coonar A. A prospective study of autologous ‘blood patch’ pleu-rodesis for persistent air leak after pulmonary resection. Eur J Cardiothorac Surg 2004;26:897-900. [Crossref] [PubMed]
- da Costa KM, Saxena AK. Thoracoscopic management of blebs: Resection with/out primary pleurodesis. Indian J Pediatr 2018;85:257-60. [Crossref] [PubMed]
- Rodgers-Fischi P, Vyas KS, Davenport D, et al. Trends in the management of sponta-neous pneumothorax: A single center experience. W V Med J 2017;113:30-5. [PubMed]
- Körner H, Andersen K, Stangeland L, et al. Surgical treatment of spontaneous pneu-mothorax by wedge resection without pleurodesis or pleurectomy. Eur J Cardiothorac Surg 1996;10:656-9. [Crossref] [PubMed]
- Park JS, Han S, Kim K, et al. Pleural abrasion for mechanical pleurodesis in surgery for primary spontaneous pneumothorax: is it effective? Surg Laparosc Endosc Percutan Tech 2012;22:62-4. [Crossref] [PubMed]
- Chen JS, Hsu HH, Kuo SW, et al. Effects of additional minocycline pleurodesis after thoracoscopic procedures for primary spontaneous pneumothorax. Chest 2004;125:50-5. [Crossref] [PubMed]
- Chen JS, Hsu HH, Chen RJ, et al. Additional minocycline pleurodesis after thoracoscopic surgery for primary spontaneous pneumothorax. Am J Respir Crit Care Med 2006;173:548-54. [Crossref] [PubMed]
- Vargas FS, Wang N, Lee H, et al. Effectiveness of bleomycin in comparison to tetracycline as pleural sclerosing agent in rabbits. Chest 1993;104:1582-4. [Crossref] [PubMed]
- Bridevaux PO, Tschopp JM, Cardillo G, et al. Short-term safety of thoracoscopic talc pleurodesis for recurrent primary spontaneous pneumothorax: A prospective European multicentre study. Eur Respir J 2011;38:770-3. [Crossref] [PubMed]
- Sudduth CL, Shinnick J, Geng Z, et al. Optimal surgical technique in spontaneous pneumothorax: A systematic review and meta-analysis. J Surg Res 2017;210:32-46. [Crossref] [PubMed]
- Sadikot RT, Greene T, Meadows K, et al. Recurrence of primary spontaneous pneumo-thorax. Thorax 1997;52:805-9. [Crossref] [PubMed]
- Conservative management of spontaneous pneumothorax. Lancet 1984;1:687-9. [PubMed]
Cite this article as: Stodghill JD, Collins DT, Mahajan AK, Khandhar SJ. Primary spontaneous pneumothorax: a pathway to practice. AME Med J 2019;4:8.

