Treatment of airway complications following lung transplantation
Airway complications following lung transplantation are associated with significant morbidity and a mortality of 2–4 percent (1,2). The incidence of lung transplant related airway complications varies widely from 2–33 percent (2,3). Airway complications are associated with significant functional impairment, poor quality of life, increased hospitalizations, and greater health care resource utilization (4,5). Overall survival rates for patients with airway complications are reduced at 30-days, 90-days, 1-year, 3-years, and 5-years (6). As improvement in surgical technique and post-transplant care have evolved, the incidence of airway complications has decreased. The incidence of airway complications is lower in heart-lung transplantations at approximately 3 to 14 percent (2). Additionally, advances that improve donor lung function, such as normothermic ex vivo lung perfusion, have shown promise for increasing allograft viability with an incidence of airway complications of only 6 percent (7).
The common airway complications causing structural and functional impairment following lung transplantation include granulation tissue formation, anastomotic stenosis, transplant related bronchomalacia, bronchial fistulae, endobronchial anastomotic infection, and anastomotic dehiscence. Management of these complications is not uniform between different physicians and various institutions. Unfortunately, randomized, prospective trials involving management of post-transplant airway complications are rare. As a result, expert opinion guides many treatment principles while standardization of airway complications management continues to evolve. This review explores the risk factors, diagnosis, and bronchoscopic management of airway complications following lung transplantation. Where literature was not available to guide management, expert opinion was utilized.
Determining the incidence of airway complications following lung transplantation is difficult due to the few accepted and validated classification systems. Couraud et al. describe a classification system focusing on the severity of airway necrosis while Shennib et al. describe a classification system based on variable degrees of anastomotic healing (2,8). These classification systems provide important descriptions of anastomotic healing with Couraud et al. providing implications for the risk of developing stenosis based on the degree of anastomotic necrosis.
Risk factors for airway complications
Common risk factors for post-lung transplant anastomotic complications include anastomotic ischemia, surgical technique, pulmonary infection, and allograft dysfunction. Anastomotic ischemia is the major driver for the development of airway complications following lung transplantation (Figure 1). Normal human lungs and airways are perfused by a dual blood supply arising from the pulmonary and bronchial circulations. During lung transplantation surgery, the bronchial arterial supplies are not typically reestablished. As a result, the relative decrease in blood supply to the anastomotic region increases the risk of ischemia. These ischemic changes result in an inflammatory cascade that may cause bronchial lumen remodeling and airway dysfunction. This region remains ischemic for weeks after transplantation as the bronchial artery circulation begins to reestablish itself through collateral formation (9,10).
The length of time the donor patient remains on mechanical ventilation prior to organ recovery is associated with the development of post-transplantation airway complications. The probability of developing an airway complication has been found to be highest for donors on mechanical ventilation between 50 and 70 hours prior to organ recovery (3,11). Rapid weaning from mechanical ventilation following transplantation is ideal to avoid the potential complications of positive pressure ventilation which include ventilator associated allograft infections and critical illness associated weakness.
The length of the donor bronchus affects the incidence and degree of airway ischemia. Anastomosis of a short donor bronchus compared to a longer donor bronchus may reduce the risk of prolonged ischemia, as less collateralization is necessary. Shortening the donor bronchus length at the lobar carina has been shown to reduce the incidence of airway complications in the first year following lung transplantation versus the standard donor airway length (2.6% vs. 11.1%) (11). Bronchial artery revascularization (BAR) is a technique that reestablishes bronchial artery circulation during lung transplantation surgery. Despite the theoretical benefits of BAR, the early outcomes with and without this procedure are comparable. Due to the complexity of BAR and the potential for increased graft ischemic time have made the performance of BAR uncommon.
A number of surgical techniques can be used for anastomosing the donor and recipient bronchi during lung transplantation. The “end-to-end” technique is performed by attaching the end of the donor bronchus to the end of the recipient bronchus without tissue overlap. The end-to-end technique is the preferred as it has been associated with fewer post-transplant airway complications (12). Conversely, a “telescoping” anastomosis is performed by overlapping the bronchi of the donor and recipient. The use of a telescoping anastomosis is associated with a 48 percent incidence of airway complication, namely anastomotic stenosis and infection (12). This technique is less commonly performed due to increased incidence of anastomotic stenosis, but may be necessary when there is a significant discrepancy between the diameters of the donor and recipient bronchi. Additionally, tissue buttressing can be performed by wrapping pleura, pericardium, or omental fat around the anastomosis.
The presence pulmonary infections, both in the pre-transplantation and post-transplantation periods, increase the risk of post-operative airway complications. Preoperative infection with Pseudomonas cepacia is particularly harmful, as the relative risk of developing an airway complication increases by 29 percent compared to non-infected patients (CI: 2.84–303.26; P=0.002) (11). Post-operative fungal infections, especially with Aspergillus Fumigatus, are associated with increased incidence of airway complications in the first 30 days after lung transplantation (13).
Development of allograft dysfunction including ischemia reperfusion injury or rejection within the first month following transplantation may be associated with increased risk of airway complications (14). A large cohort of 9,335 patients found to have a significant association of bronchial strictures with the presence of acute rejection in the first post-operative year (15). Additional studies will be needed to determine additional association of allograft dysfunction with airway complications.
Types of airway complications
Granulation tissue formation
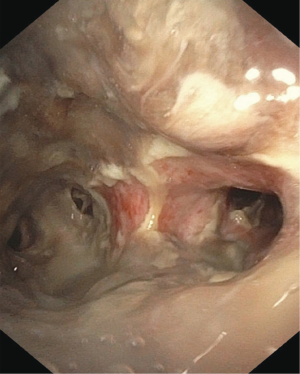
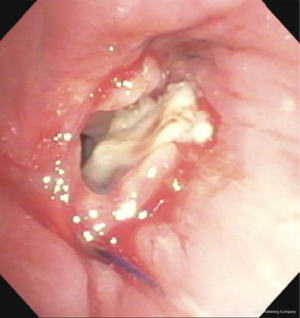
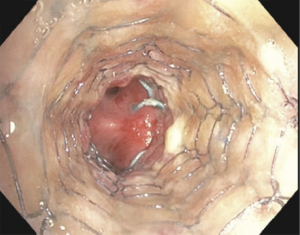
Approximately 20% of patients develop obstructive granulation tissue within months of lung transplantation (16) (Figure 2). While a number of risk factors may lead to the development of excess granulation tissue, Aspergillus infection at the surgical anastomosis is a common risk factor (17). The presence of obstructive granulation tissue may lead to decreased pulmonary function and post-obstructive pneumonia.
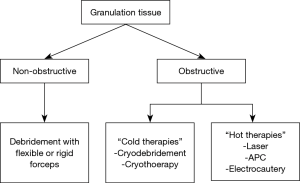
A number of treatment options exist for the treatment of post-transplant granulation tissue formation. The type of intervention utilized depends on the presence of an obstructive or non-obstructive pattern (18) (Figure 3). Non-obstructive granulation tissue is best treated with mechanical debridement via flexible forceps or rigid forceps. Mechanical debridement is effective and is associated with presumably less trauma compared to electrosurgical therapies, such as laser, electrocautery, argon plasma coagulation (APC), or radiofrequency ablation (RFA). Trauma to the anastomosis that can occur with the use of electrosurgical tools can result in excess inflammatory and development of even more granulation tissue. Obstructive granulation tissue may require more extensive debridement to regain patency of the airway. In the authors’ opinion, utilization of cold therapies such as Cryodebridement and cryotherapy can be extremely effective in these situations by offering the capabilities to debulk granulation tissue with a decreased risk of granulation tissue reformation. Further research is needed to determine the best approaches to treat obstructing granulation tissue formation in the post-lung transplant patient
Bronchial stenosis
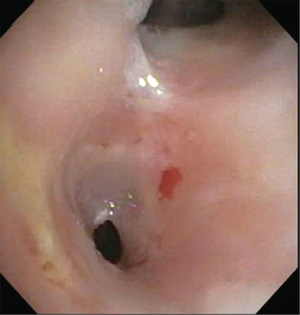
Anastomotic stenosis is the most common airway complication following lung transplantation and is seen in approximately 1.6–32 percent of patients (12,13,19) (Figure 4). Even patients undergoing lung retransplantation, the incidence of bronchial stenosis remains at 7.9 percent in one study (20). While bronchial stenosis can develop at any time following transplantation, it is most commonly seen within the first 2–9 months (21,22). The development of an anastomotic stenosis has been shown to be an independent risk factor for death following lung transplantation (15). Risk factors for bronchial stenosis include anastomotic ischemia, severe reperfusion edema, and early rejection (14).
The types of bronchial stenosis following lung transplantation can be separated into two categories: anastomotic stenosis and non-anastomotic stenosis. Anastomotic stenosis occurs at the donor and recipient surgical site and is more common. Non-anastomotic stenosis develops more distal to the surgical anastomosis (23). Both types of complications can lead to a reduction in pulmonary function and post-obstructive pneumonia.
Management of post-transplant stenosis can be challenging and may involve repeated bronchoscopic interventions. Initial treatment of anastomotic stenoses typically involve balloon bronchoplasty using controlled radial expansion balloons (CRE), sometimes in conjunction with radial incisions at the site of the stenosis. The addition of radial airway incisions can be created via cutting balloon or electrocautery knife. These scores in the stenosis function to allow the tissue to tear when dilated with the CRE balloon. In the authors’ opinion, this technique can improve the chances of long-term resolution of a web-like airway stenosis.
Airway stenoses refractory to balloon dilation with or without endobronchial incisions may require airway stent placement (Figure 5). Patients with bronchial stenosis demonstrated a mean survival of 82 versus 22 months, respectively, in patients undergoing stent placement versus those treated with simple bronchoplasty alone (24). The goal of stent placement is to restore near physiologic patency to the transplanted airway. Additionally, the presence of an airway stent can result in endobronchial remodeling with resolution of the stenosis over time. Unfortunately, patients can exhibit significant airway malacia after the stent is removed despite resolution of the stenosis.
The most common airway stents used to treat post-transplant stenoses include silicone stents and self-expanding metallic stents (SEMS). While advantages and disadvantages to each type of airway stent is beyond the scope of this paper, silicone stents are often favored as they can be easily removed from the airway without significant scar tissue formation. While both silicone stents and SEMS have been traditionally used in the mainstem bronchi, studies promoting the use of silicone Y-stents and Atrium iCast metallic stents in the lobar bronchus have gained attention (25,26). Such approaches to lobar salvage in the setting of lobar airway stenosis provide an effective alternative to repeated balloon dilations.
Post-transplant bronchomalacia
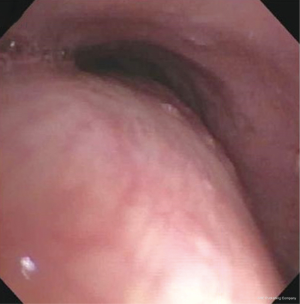
Transplant related bronchomalacia is a condition characterized by excessive bowing of the posterior tracheal or bronchial membrane during expiration at the site of the surgical anastomosis (Figure 6). Mild bowing of the posterior membrane is normal, but a greater than 50 percent decrease in the luminal diameter on expiration is considered diagnostic of significant bronchomalacia, especially when dyspnea is present on exertion or at rest (27). Symptoms of transplant related bronchomalacia include shortness of breath, cough, and recurrent respiratory infections.
The incidence of bronchomalacia in lung transplant patients is difficult to determine, but has been reported at 1-4 percent in single-center studies (28). The cause of transplant related bronchomalacia is poorly understood, although the presence of bronchiolitis obliterans (BOS) has been associated with the condition (29). Bronchomalacia related to lung transplantation can be classified as perianastomotic or distal. Perianastomotic malacia is present 1centimeter proximal or distal to the bronchial anastomosis while distal malacia is located greater than 1cm distal to the anastomosis.
Diagnosis of both transplant related bronchomalacia requires bronchoscopic visualization of the airways during dynamic airway maneuvers (30). Dynamic airway maneuvers require the patient to be under light sedation and follow commands to inhale and forcibly exhale while the bronchoscope is within the tracheobronchial tree. The presence of posterior membrane bowing resulting in greater than 50 percent obstruction during dynamic airway maneuvers is diagnostic of significant malacia if clinical symptoms are present.
Management of bronchomalacia following lung transplantation depends on the degree of dyspnea experienced and if the patient has suffered from recurrent bouts of pneumonia. Patients with minimal clinical symptoms may benefit from aggressive pulmonary hygiene including mucolytics and noninvasive continuous positive airway pressure (CPAP) ventilation when sleeping. Severe malacia following lung transplant with functional impairment often requires stent placement. The authors prefer leaving silicone stents in airways with significant malacia for 9–12 months to promote airway remodeling. Unfortunately, in the absence of airway remodeling the stents may need to be left in permanently.
Bronchial fistulae
Three types of bronchial fistulae can develop following lung transplantation: bronchopleural fistulae, bronchomediastinal fistulae, and bronchovascular fistulae. These pathologic communications are likely due to significant and prolonged bronchial ischemia following lung transplantation (31).
Bronchopleural fistulae may present with shortness of breath, hypotension, subcutaneous emphysema, or pneumothorax with or without a persistent airleak. Tube thoracostomy is the first step for treatment of bronchopleural fistulas when a pneumothorax is present. Additionally, broad-spectrum antibiotic and anti-fungal medications should be implemented. Surgical options in appropriate patients include chronic open drainage, direct closure with flap reinforcement, trans-sternal bronchial closure, or thoracoplasty (32). Bronchoscopic interventions include the use of fibrin glue for small defects or metallic stents for larger, proximal fistulas. The use of metallic uncovered stents promotes granulation formation at the site of the defect to help seal small fistulae and can be removed after 4–6 weeks.
Bronchomediastinal fistulae often present as mediastinal infections. While aerosolized antibiotic therapy is the standard of care for small bronchomediastinal fistulas, if severe infection is present mediastinal debridement may be necessary.
The development of a bronchovascular fistula is associated with significant mortality. Clinical manifestations of fistula formation between the mainstem bronchus with the pulmonary artery, pulmonary vein, aorta, or left atrium typically result in hemoptysis followed by massive hemorrhage (31). While few interventions are available for the treatment of bronchovascular fistulas, successful management has been reported involving bilobectomy or pneumonectomy (32,33).
Anastomotic dehiscence
Anastomotic dehiscence is the separation of the surgical anastomosis due to extreme necrosis (34). This complication following lung transplantation is associated with significant morbidity and mortality (35). The incidence of severe, clinically relevant dehiscence is estimated at 2 percent (36). Risk factors associated with airway anastomotic dehiscence include airway ischemia, high-dose perioperative steroids, acute rejection, immunosuppression, and infection, particularly Aspergillus species (13,18). Common presenting symptoms of anastomotic dehiscence include shortness of breath, pneumothorax with persistent airleak, pneumomediastinum, and the inability to wean from the ventilator.
Bronchoscopic visualization of an anastomotic dehiscence is necessary to confirm the diagnosis and assess the severity of the defect. Management of an anastomotic dehiscence is dependent on symptoms and severity. Partial dehiscence is often treated conservatively with close bronchoscopic surveillance along with antibiotic therapy, both intravenous and inhaled (30). Management of a severe anastomotic dehiscence is treated with temporary placement of an uncovered SEMS over the defect (37). Utilization of uncovered SEMS provides a lattice for granulation formation and airway remodeling. The uncovered SEMS used to a treat severe dehiscence are typically removed after 4–6 weeks with great caution so as not to create a larger defect during extraction.
Surgical intervention for severe dehiscence may be required if bronchoscopic treatments are not effective. Common surgical treatments for severe dehiscence include open repair for re-anastomosis, flap bronchoplasty, transplant pneumonectomy, or re-transplantation (22).
Anastomotic infection
While anastomotic fungal infections are rare in lung transplant patients, they can prove fatal. Exposure of the transplanted lung to the outside environment along with significant immunosuppression increases the risk for opportunistic infections. The short period of anastomotic ischemia following transplantation, disruption of lymphatic drainage, altered alveolar phagocytic function, and impairment of mucociliary clearance increase the risk of anastomotic saprophytic infections in lung transplant patients (38). Fungal infections are seen in 15 to 35 percent of lung transplant recipients, with Aspergillus species and Candida species being responsible for 80 percent of these infections (39). Determining if fungal or bacterial species identified from bronchoscopy in lung transplant patients can be challenging. Signs and symptoms of anastomotic infections include fevers, cough, hemoptysis, wheezing, and changes in spirometry. Unfortunately, anastomotic infectious can also be asymptomatic. Brushings and biopsies from the anastomosis sent for cultures are the tests of choice for diagnosing anastomotic infections.
Antifungal prophylaxis is important preventative measure for anastomotic infections, along with early treatment with broad-spectrum therapy for any infection identified from bronchoscopy.
Summary
While the incidence of airway complications following lung transplantation is low, the associated morbidity is significant. Granulation tissue formation, anastomotic stenosis, transplant related bronchomalacia, airway fistulization, airway dehiscence, and anastomotic infections are airway complications that can make a newly transplanted lung less functional. Patients suffer from recurrent bronchoscopies and hospitalizations related to these complications. Although bronchoscopic interventions are ideal for treatment, some patients require surgical intervention or even repeat transplantation. The body of literature related to the management of airway complications following lung transplantation continues to grow, but further research is necessary to elucidate the best care for these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.01.06). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. Dr. Mahajan served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from Aug 2017 to Dec 2020. Dr. Khandhar served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Date H, Trulock EP, Arcidi JM, et al. Improved airway healing after lung transplantation. An analysis of 348 bronchial anastomoses. J Thorac Cardiovasc Surg 1995;110:1424-32; discussion 1432-3. [Crossref] [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [Crossref] [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk Factors for Airway Complications Within the First Year After Lung Transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Dezfouli AA, Najafizadeh K, Parsa T, et al. Postlung transplant rehospitalization: a study of causes, health care burden, and outcomes. Exp Clin Transplant 2009;7:192-6. [PubMed]
- Seiler A, Jenewein J, Martin-Soelch C, et al. Post-transplant outcome-clusters of psychological distress and health-related quality of life in lung transplant recipients. Swiss Med Wkly 2015;145:w14236 [PubMed]
- Awori Hayanga JW, Aboagye JK, Shigemura N, et al. Airway complications after lung transplantation: Contemporary survival and outcomes. J Heart Lung Transplant 2016;35:1206-11. [Crossref] [PubMed]
- Cypel M, Aigner C, Sage E, et al. Three Center Experience with Clinical Normothermic Ex Vivo Lung Perfusion. J Heart Lung Transplant 2013;32:S16. [Crossref]
- Couraud L, Nashef SA, Nicolini P, et al. Classification of airway anastomotic healing. Eur J Cardiothorac Surg 1992;6:496-7. [Crossref] [PubMed]
- Pearson FG, Goldberg M, Stone RM, et al. Bronchial Arterial Circulation Restored After Reimplantation of Canine Lung. Can J Surg 1970;13:243-50. [PubMed]
- Siegelman SS, Hagstrom JW, Koemer SK, et al. Restoration of Bronchial Artery Circulation After Canine Lung Allotrasplantation. J Thorac Cardiovasc Surg 1977;73:792-5. [PubMed]
- Choong CK, Sweet SC, Zoole JB, et al. Bronchial airway anastomotic complications after pediatric lung transplantation: incidence, cause, management, and outcome. J Thorac Cardiovasc Surg. 2006;131:198-203. [Crossref] [PubMed]
- Garfein ES, McGregor CC, Galantowicz ME, Schulman LL. Deleterious effects of telescoped bronchial anastomosis in single and bilateral lung transplantation. Ann Transplant 2000;5:5-11. [PubMed]
- Herrera JM, McNeil KD, Higgins RS, et al. Airway complications after lung transplantation: treatment and long-term outcomes. Ann Thorac Surg 2001;71:989-93. [Crossref] [PubMed]
- Ruttmann E, Ulmer H, Marchese M, et al. Evaluation of factor damaging the bronchial wall in lung transplantation. J Heart Lung Transplant 2005;24:275-81. [Crossref] [PubMed]
- Castleberry AW, Worni M, Kuchibhatia M, et al. A comparative analysis of bronchial stricture after lung transplant in recipients with and without early acute rejection. Ann Thorac Surg 2013;96:1008-17. [Crossref] [PubMed]
- Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys 2008;70:701-6. [Crossref] [PubMed]
- Nathan SD, Shorr AF, Schmidt ME, et al. Aspergillus and endobronchial abnormalities in lung transplant recipients. Chest 2000;118:403-7. [Crossref] [PubMed]
- Mahajan AK, Folch E, Khandhar SJ, et al. Airway Complications Following Lung Transplantation: A Comprehensive Review. Chest 2017;152:627-38. [Crossref] [PubMed]
- De Gracia J, Culebras M, Alvarez A, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33. [Crossref] [PubMed]
- Halloran K, Keshavjee S, Chaparro C, et al. Clinical Outcomes in Lung Retransplantation. J Heart Lung Transplant 2014;33:S297. [Crossref]
- Dutau H, Cavailles A, Sakr L, et al. A retrospective study of silicone stent placement for management of anastomotic airway complications in lung transplant recipients: short- and long-term outcomes. J Heart Lung Transplant 2010;29:658-64. [Crossref] [PubMed]
- Moreno P, Alvarez A, Algar FJ, et al. Incidence, management and clinical outcomes of patients with airway complications following lung transplantation. Eur J Cardiothorac Surg 2008;34:1198-205. [Crossref] [PubMed]
- Hasegawa T, Iacono AT, Orons PD, et al. Segmental nonanastomotic bronchial stenosis after lung transplantation. Ann Thorac Surg 2000;69:1020-4. [Crossref] [PubMed]
- Abi-Jaoudeh N, Francois RJ, Oliva VL, et al. Endobronchial dilation for the management of bronchial stenosis in patients after lung transplantation: effect of stent placement on survival. J Vasc Interv Radiol 2009;20:912-20. [Crossref] [PubMed]
- Sethi S, Wang J, Machuzak M, et al. Clinical Success Stenting Lobar and Segmental Bronchi for “Lobar Salvage” in Bronchial Stenosis. Chest 2014;146:732A. [Crossref]
- Lee HJ, Puchalski J, Sterman DH, et al. Secondary carina Y-stent placement for post-lung-transplant bronchial stenosis. J Bronchology Interv Pulmonol 2012;19:109-14. [Crossref] [PubMed]
- Ridge CA, O’Donnell CR, Lee EY, et al. Tracheobronchomalacia: current concepts and controversies. J Thorac Imaging 2011;26:278-89. [Crossref] [PubMed]
- Simoff MJ, Sterman DH, Ernst A, editors. Thoracic endoscopy advances in interventional pulmonology. Malden, MA: Blackwell Publishing; 2006.
- Novick RJ, Ahmad D, Menkis AH, et al. The importance of acquired diffuse bronchomalacia in heart-lung transplant recipients with obliterative bronchiolitis. J Thorac Cardiovasc Surg 1991;101:643-8. [PubMed]
- Carden KA, Boiselle PM, Waltz DA, et al. Tracheomalacia and tracheobronchomalacia in children and adults. Chest 2005;127:984-1005. [Crossref] [PubMed]
- Hoff SJ, Johnson JE, Frist WH. Aortobronchial fistula after unilateral lung transplantation. Ann Thorac Surg 1993;56:1402-3. [Crossref] [PubMed]
- Guth S, Mayer E, Fischer B, et al. Bilobectomy for massive hemoptysis after bilateral lung transplantation. J Thorac Cardiovasc Surg 2001;121:1194-5. [Crossref] [PubMed]
- Rea F, Marulli G, Loy M, et al. Salvage right pneumonectomy in a patient with bronchial-pulmonary artery fistula after bilateral sequential lung transplantation. J Heart Lung Transplant 2006;25:1383-6. [Crossref] [PubMed]
- Weder W, Inci I, Korom S, et al. Airway complications after lung transplantation: Risk factors, prevention and outcome. Eur J Cardiothorac Surg 2009;35:293-8; discussion 298. [Crossref] [PubMed]
- Kirk AJ, Conacher ID, Corris PA, et al. Successful surgical management of bronchial dehiscence after single-lung transplantation. Ann Thorac Surg 1990;49:147-9. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Chang CC, Hsu HH, Kuo SW, et al. Bronchoscopic gluing for post-lung-transplant bronchopleural fistula. Eur J Cardiothorac Surg 2007;31:328-30. [Crossref] [PubMed]
- Pinel C, Fricker-Hidalgo H, Lebeau B, et al. Detection of circulating Aspergillus fumigatus galactomannan: value and limits of the Platelia test for diagnosing invasive aspergillosis. J Clin Microbiol 2003;41:2184-6. [Crossref] [PubMed]
- Solé A, Salavert M. Fungal infections after lung transplantation. Transplant Rev (Orlando) 2008;22:89-104. [Crossref] [PubMed]
Cite this article as: Mahajan AK, Khandhar SJ. Treatment of airway complications following lung transplantation. AME Med J 2019;4:13.