
Beneficial effect of antioxidant therapy on sperm DNA integrity is not associated with a similar effect on sperm chromatin integrity
Introduction
The rationale for using oral antioxidant supplements in male infertility stems from the understanding that semen oxidative stress (OS), through generation of reactive oxygen species (ROS), is one of the primary factors leading to male infertility and sperm abnormalities (1-4). Spermatozoa are particularly susceptible to OS due to the abundance of plasma membrane polyunsaturated fatty acids (5-7). These unsaturated fatty acids provide fluidity for sperm motility but predispose sperm to free radical attack resulting in lipid peroxidation, sperm dysfunction and oxidative damage to the DNA (8,9).
Although seminal plasma contains a number of enzymatic (superoxide dismutase, catalase, glutathione peroxidase (1,5,10,11) and non-enzymatic antioxidants (ascorbic acid, α-tocopherol, pyruvate, glutathione, L-carnitine, taurine, hypotaurine), seminal antioxidant activity may be suppressed in infertile men with seminal OS (12-15). To date, there is some controversy as to whether the high ROS levels detected in the semen of infertile men are due to increased ROS production, decreased ROS scavenging capacity or both (13,16). If we assume that high semen ROS levels are due, at least in part, to a decreased semen antioxidant scavenging capacity, the use of dietary antioxidant supplementation may be beneficial to infertile men. This concept is supported by studies showing that oral intake of antioxidants will increase semen antioxidant levels and sperm DNA quality (17-19).
Aitken et al. have proposed a 2-step model for sperm DNA fragmentation (20). In the model, the 1st step in the “development” of DNA fragmentation is defective spermatogenesis characterized by the production of immature sperm having poor chromatin compaction and the second step is oxidative injury to the DNA of poorly compacted sperm nuclei. Based on this model, we wanted to explore the potential effect of an oral antioxidant supplement on sperm chromatin compaction and DNA fragmentation. Only one study has examined both of these parameters after antioxidant therapy and has reported that antioxidants have a beneficial effect on sperm DNA fragmentation but a detrimental effect on chromatin compaction (21).
Methods
Materials
The APO-Direct kit was purchased from BD Pharmingen, CA, USA. Unless otherwise stated, all other chemicals were obtained from Sigma Chemical Co (St. Louis, MO, USA) and were at least of reagent grade.
Patient population
We conducted a retrospective study of 17 consecutive infertile men with abnormal semen parameters (reduced sperm concentration and/or motility) that were treated with an oral multivitamin (antioxidant) supplement and re-evaluated with a follow-up semen analysis. In these 17 men, sperm DNA integrity and chromatin compaction were assessed before and 3 months after initiating antioxidant treatment. These men were seen for infertility evaluation at the OVO fertility clinic in Montreal, Canada between May 2016 and November 2017. The men in this study received the Fertil Pro antioxidant supplement with the following daily dose: 400 mg L carnitine, 300 mg vitamin C, 100 mg coenzyme Q10, 67 mg vitamin E, 30 mg zinc, 3 mg beta-carotene, 2 mg lycopene, 1 mg folic acid, 50 µg vitamin B12, 30 µg selenium and 25 µg vitamin D.
None of these men had azoospermia, severe oligozoospermia (<5 million sperm/mL), complete asthenozoospermia or evidence of genital tract infection (none had clinical symptoms of genital tract infection or leukocytospermia). Men exposed to gonadotoxins (e.g., smokers, recreational drug users and alcoholics) were excluded. None of the men had a clinical varicocele or an exam suggestive of genital tract inflammation (tender vas and/or epididymis). None of the men were selected based on the results of sperm DNA test result. Sperm morphology scores were not included in this study because this parameter was not assessed in conjunction with the other parameters (sperm DNA integrity and chromatin compaction).
Consent was not obtained from patients. The OVO research and development scientific committee reviewed our study and we received approval as a quality control study. We followed the principles of the Helsinki Declaration.
Semen handling
Samples were obtained by masturbation after 2–3 days of sexual abstinence. After liquefaction of semen, standard semen parameters (volume, concentration, motility) were obtained using a computer-assisted semen analyzer (CASA; Hamilton Thorne, Beverly, MA, USA). All of the semen samples had motile spermatozoa and none had significant numbers of round cells or leukocytospermia as per WHO guidelines (all had <1 million round cells/mL).
Following liquefaction, two aliquots of semen containing approximately 1 million spermatozoa were collected from the original sample and fixed in 2% para-formaldehyde. The samples were then stored overnight at –20 °C in 70% ethanol for later evaluation of sperm DNA fragmentation by terminal deoxynucleotidyl transferase 2'-deoxyuridine 5'-triphosphate (dUTP) nick end labeling (TUNEL) assay. A separate 25–100 µL aliquot of fresh semen was collected and frozen at –70 °C for later sperm cytochemical chromatin testing [% aniline blue (AB) staining].
Sperm DNA fragmentation by TUNEL assay
Sperm DNA damage was assessed by flow cytometry-based TUNEL assay and reported as DNA fragmentation index (%DFI)—reflecting the percentage of cells with DNA damage, as described previously (22). A fluorescein isothiocyanate—dUTP kit was used according to the instructions of the manufacturer for flow cytometry technique. Briefly, whole semen samples were fixed with a formaldehyde solution, centrifuged 5 min at 300 × g and washed in phosphate buffered saline (PBS). The samples were subsequently stored in ice cold 70% ethanol at ‒20 °C for a minimum of 12–18 h before testing. After washing, the labeling reaction (supplied with the In Situ Cell Death Detection Kit, Fluorescein) was performed by incubating sperm in 50 µL of labeling solution containing TdT enzyme for 1 h at 37 °C in the dark. Finally, samples were rinsed, resuspended in PBS and analyzed by flow cytometry. For each sample, a negative control (omitting TdT) was performed. At the beginning of each series of samples, a positive control was performed (obtained by treating sperm with DNase diluted in PBS).
Flow cytometry was used to detect TUNEL staining of sperm from patients with >1 million cells per milliliter. For each sample, at least 10,000 events were recorded within the region characteristic of spermatozoa, using a flow cytometer (BD Accuri C6, Becton Dickinson, CA, USA) equipped with two lasers detectors FL1 (488 nm) with a standard 533/30 band pass (BP) that detects green fluorescence fluorescein isothiocyanate (FITC) and FL2 with standard 675/25 nm BP that detects red or propidium iodide (PI) fluorochrome. The amount of sperm DNA fragmentation was determined as the percentage of sperm having fluorescence intensities above a threshold established in the negative control histogram. We have shown that repeat testing using our flow cytometry TUNEL assay gives comparable results with a low (<10%) intra-assay variability (data not shown).
Cytochemical test of sperm chromatin compaction: AB
Thawed semen samples were fixed with 70% ethanol and kept at −20 °C before further processing. Smears were prepared from the fixed semen samples, left to air-dry at 20 °C for 30 min and immediately stained. For AB staining, smears are incubated with the dye (5% AB in 4% acetic acid) for 5 min, washed 3 times with dH2O and mounted with glycerol (23,24).
We counted at least 200 spermatozoa per slide and followed the same grading system as previously reported (23,24). We categorized the spermatozoa into one of three groups: dark blue (dark blue stain over the whole head), pale (light blue staining of the entire head) or intermediate staining (dark blue staining of the post-acrosomal region only). For the purpose of this study we reported positive staining as the percentage of cells with dark blue staining (AB). We have previously found that the AB inter-assay variability is low (data not shown) and testing of fresh and frozen samples yielded comparable results (25).
Data analysis
Results are expressed as means ± one standard error (SE). Differences between the pre- and 3-month post-antioxidant supplementation parameters were estimated by Wilcoxon signed-ranks test (the Wilcoxon signed-ranks test was used because the populations-values were not normally distributed). The calculations of correlation coefficients between parameters (variables) were performed using a nonparametric procedure, the Spearman rank-order correlation. All hypothesis testing was two-sided with a probability value of 0.05 deemed as significant. Analyses were conducted using the sigma stat program [Statistical Package for the Social Sciences (SPSS), Chicago, IL, USA).
Results
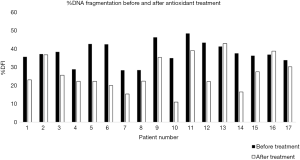
We identified 17 consecutive infertile men with reduced sperm concentration (<15 million/mL) and/or progressive motility (<32%) that were treated an oral antioxidant supplement. These men had sperm DNA testing and sperm chromatin compaction assessment before and 3 months after treatment. The baseline (pre-treatment) sperm %DFI was 38.6% (Table 1).
Table 1
| Parameter | Pre-therapy | Post-therapy (3-months) | P value |
|---|---|---|---|
| Sperm concentration (×106/mL) | 50±1.8 | 61±1.8 | 0.34a |
| Progressive motility (%) | 49±1.4 | 52±1.4 | 0.38a |
| Sperm DNA fragmentation (%) | 38.6±1.7 | 26.6±1.8 | <0.0001a |
| Percent positive aniline blue stain | 28.8±3.2 | 30.1±2.8 | 0.48a |
Values are means ± SD. a, Wilcoxon signed-ranks test. SE, standard error.
Antioxidant therapy was associated with a significant decline in %DFI (from 38.6%±1.7% to 26.6%±1.8%, P<0.0001), with the majority of patients (94%) experiencing a diminution in their %DFI after therapy (Figure 1). Furthermore, nearly half of the men (8/17) with an elevated initial %DFI (>30%) had a %DFI below 30% after therapy. In contrast, antioxidant therapy was not associated with a significant improvement in sperm concentration or progressive motility (Table 1). Moreover, antioxidant therapy was also not associated with a significant reduction in % positive AB staining (Table 1). There were no significant correlations between sperm DNA fragmentation, sperm chromatin compaction, sperm concentration and progressive motility (data not shown).
Discussion
Treatment of OS should first involve strategies to reduce or eliminate stress-provoking conditions including smoking, varicocele, genital infection, gonadotoxins and hyperthermia (26). The rationale for treating infertile men with oral antioxidants is based on the premise that seminal OS (common in infertile men) is due in part to a deficiency in seminal antioxidants. The practice of prescribing oral antioxidant is supported by the lack of serious side effects related to antioxidant therapy, although, few studies have carefully evaluated the risk of over-treatment with antioxidants (27). Ideally, an oral antioxidant should reach high concentrations in the reproductive tract and replete a deficiency of vital elements important for spermatogenesis. Additionally, the antioxidant supplement should augment the scavenging capacity of seminal plasma and reduce the levels of semen ROS (1). However, the levels of semen ROS should not be entirely suppressed (by oral antioxidants) as this may impair normal sperm functions (e.g., sperm capacitation and hyperactivation) that normally require low levels of ROS (28-30).
In this study, we have treated a group of infertile men with an oral, multivitamin supplement for three months and have observed that this treatment was associated with a significant decrease in sperm DNA fragmentation. Close to 100% of the men experienced a reduction in sperm %DFI after antioxidant therapy. However, antioxidant treatment was not associated with significant beneficial changes in conventional semen parameters (concentration and progressive motility). Moreover, antioxidant treatment was also not associated with a significant improvement in sperm chromatin integrity.
Our findings demonstrate that the improvement in sperm DNA fragmentation associated with antioxidant therapy was not accompanied by a parallel improvement in sperm chromatin compaction, an observation that might appear contradictory at first glance. The observations on the protective role of antioxidants on sperm DNA fragmentation suggest that this fragmentation occurs at least in part as a result of OS, as shown in previous studies (1-3,17). However, our results on sperm chromatin compaction suggest that this characteristic is not influenced by antioxidant treatment in the way that DNA fragmentation is. The proper assembly and compaction of sperm chromatin depends on the fidelity of spermiogenesis (i.e., the proper replacement of histones by protamines) and on sperm maturation in the post-testicular (e.g., epididymal) environment (20,31). One of the final steps in sperm chromatin compaction in the testis and in the post-testicular environment involves the formation of inter- and intra-molecular protamine bonds, a process that requires a mild OS (32). It is not surprising to observe that antioxidants do not improve sperm chromatin compaction as the use of oral antioxidants may interfere with proper chromatin compaction by dampening the OS necessary for this event. Moreover, there is no plausible way to explain how mechanistically oral antioxidants could correct the incomplete replacement of histones by protamines and measurably improve spermatogenesis.
The improvement in sperm DNA integrity after antioxidant therapy is more credible than changes in conventional sperm parameters because measures of sperm DNA damage exhibit a lower degree of biologic variability than standard semen parameters (33-36). Nonetheless, it is important to note that there are possible confounders, such as, changes in lifestyle or social habits, that may have influenced the results of this study. We also recognize that the results of our study would be strengthened had we conducted a controlled or randomized controlled trial (RCT) of antioxidant therapy. A small number of studies have evaluated the role of antioxidants on sperm DNA damage and have generally shown improved DNA integrity after therapy (3,17-19,37). Only one published study has examined the effect of antioxidants on sperm chromatin integrity and has reported an adverse effect of this therapy on sperm chromatin compaction using high DNA stainability (measured by SCSA) as a measure of chromatin compaction (21).
To date, over 100 clinical and experimental studies have examined the effect of oral antioxidants on sperm parameters. Despite this large body of literature, it is not possible to establish firm conclusions regarding the optimal antioxidant treatment for infertile men because the published studies report on different types and doses of antioxidants, the studies are small with variable populations of infertile patients, the end-points vary and few of the studies are placebo-controlled (1,38-40). A systematic review of randomized trials has concluded that antioxidant therapy for male infertility may improve semen parameters and pregnancy outcomes, but the heterogeneity of the studies is such that it is not possible to establish firm conclusions about the value of this therapy (41). As such, the true benefit of oral antioxidant therapy remains controversial because the mechanism of action of antioxidants in the treatment of male infertility (i.e., suppression of seminal OS) has not been confirmed and many of these studies are small with variable treatment regimens.
In conclusion, in this retrospective study of infertile men with idiopathic infertility, we have shown that an oral antioxidant supplement is associated with a significant improvement in sperm DNA integrity but not chromatin compaction. These data demonstrate the complex nature of sperm chromatin and the differential effect of antioxidants on various aspects of sperm chromatin structure.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.06.01). A Zini, F Bissonnette and J Kadoch are shareholders in YAD-Tech (this company manufactures the antioxidant vitamin used in this study). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Consent was not obtained from patients. The OVO research and development scientific committee reviewed the study and authors received approval as a quality control study. This study followed the principles of the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zini A, San Gabriel M, Baazeem A. Antioxidants and sperm DNA damage: a clinical perspective. J Assist Reprod Genet 2009;26:427-32. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN, Finnie JM, et al. Analysis of the relationships between oxidative stress, DNA damage and sperm vitality in a patient population: development of diagnostic criteria. Hum Reprod 2010;25:2415-26. [Crossref] [PubMed]
- Fraga CG, Motchnik PA, Shigenaga MK, et al. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci U S A 1991;88:11003-6. [Crossref] [PubMed]
- Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril 1992;57:409-16. [Crossref] [PubMed]
- Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987;81:459-69. [Crossref] [PubMed]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. I. Effects on the motility of intact spermatozoa and on sperm axonemes. J Androl 1992;13:368-78. [PubMed]
- Zini A, Garrels K, Phang D. Antioxidant activity in the semen of fertile and infertile men. Urology 2000;55:922-6. [Crossref] [PubMed]
- Alvarez JG, Touchstone JC, Blasco L, et al. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl 1987;8:338-48. [Crossref] [PubMed]
- Twigg J, Fulton N, Gomez E, et al. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod 1998;13:1429-36. [Crossref] [PubMed]
- Chabory E, Damon C, Lenoir A, et al. Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice. J Clin Invest 2009;119:2074-85. [PubMed]
- Weir CP, Robaire B. Spermatozoa have decreased antioxidant enzymatic capacity and increased reactive oxygen species production during aging in the Brown Norway rat. J Androl 2007;28:229-40. [Crossref] [PubMed]
- Smith R, Vantman D, Ponce J, et al. Total antioxidant capacity of human seminal plasma. Hum Reprod 1996;11:1655-60. [Crossref] [PubMed]
- Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 1993;16:183-8. [Crossref] [PubMed]
- Kobayashi T, Miyazaki T, Natori M, et al. Protective role of superoxide dismutase in human sperm motility:superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod 1991;6:987-91. [Crossref] [PubMed]
- Thiele JJ, Friesleben HJ, Fuchs J, et al. Ascorbic acid and urate in human seminal plasma: determination and interrelationships with chemiluminescence in washed semen. Hum Reprod 1995;10:110-5. [Crossref] [PubMed]
- Lewis SE, Boyle PM, McKinney KA, et al. Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril 1995;64:868-70. [Crossref] [PubMed]
- Kodama H, Yamaguchi R, Fukuda J, et al. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil Steril 1997;68:519-24. [Crossref] [PubMed]
- Greco E, Iacobelli M, Rienzi L, et al. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J Androl 2005;26:349-53. [Crossref] [PubMed]
- Martínez-Soto JC, Domingo JC, Cordobilla B, et al. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst Biol Reprod Med 2016;62:387-95. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 2010;16:3-13. [Crossref] [PubMed]
- Ménézo YJ, Hazout A, Panteix G, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online 2007;14:418-21. [Crossref] [PubMed]
- Sharma RK, Sabanegh E, Mahfouz R, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010;76:1380-6. [Crossref] [PubMed]
- de Lamirande E, Gagnon C. Paradoxical effect of reagents for sulfhydryl and disulfide groups on human sperm capacitation and superoxide production. Free Radic Biol Med 1998;25:803-17. [Crossref] [PubMed]
- de Lamirande E, San Gabriel MC, Zini A. Human sperm chromatin undergoes physiological remodeling during in vitro capacitation and acrosome reaction. J Androl 2012;33:1025-35. [Crossref] [PubMed]
- Alkhayal A, San Gabriel M, Zeidan K, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet 2013;30:1519-24. [Crossref] [PubMed]
- Pan MM, Hockenberry MS, Kirby EW, et al. Male Infertility Diagnosis and Treatment in the Era of In Vitro Fertilization and Intracytoplasmic Sperm Injection. Med Clin North Am 2018;102:337-47. [Crossref] [PubMed]
- Biesalski HK, Tinz J. Multivitamin/mineral supplements: Rationale and safety. Nutrition 2017;36:60-6. [Crossref] [PubMed]
- Aitken RJ, Paterson M, Fisher H, et al. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci 1995;108:2017-25. [PubMed]
- Griveau JF, Le Lannou D. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl 1997;20:61-9. [Crossref] [PubMed]
- de Lamirande E, Jiang H, Zini A, et al. Reactive oxygen species and sperm physiology. Rev Reprod 1997;2:48-54. [Crossref] [PubMed]
- Oliva R. Protamines and male infertility. Hum Reprod Update 2006;12:417-35. [Crossref] [PubMed]
- Kosower NS, Katayose H, Yanagimachi R. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J Androl 1992;13:342-8. [PubMed]
- Evenson DP, Jost LK, Baer RK, et al. Individuality of DNA denaturation patterns in human sperm as measured by the sperm chromatin structure assay. Reprod Toxicol 1991;5:115-25. [Crossref] [PubMed]
- Zini A, Kamal K, Phang D, et al. Biologic variability of sperm DNA denaturation in infertile men. Urology 2001;58:258-61. [Crossref] [PubMed]
- Smit M, Dohle GR, Hop WC, et al. Clinical correlates of the biological variation of sperm DNA fragmentation in infertile men attending an andrology outpatient clinic. Int J Androl 2007;30:48-55. [Crossref] [PubMed]
- Oleszczuk K, Giwercman A, Bungum M. Intra-individual variation of the sperm chromatin structure assay DNA fragmentation index in men from infertile couples. Hum Reprod 2011;26:3244-8. [Crossref] [PubMed]
- Tunc O, Thompson J, Tremellen K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod Biomed Online 2009;18:761-8. [Crossref] [PubMed]
- Agarwal A, Nallella KP, Allamaneni SS, et al. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online 2004;8:616-27. [Crossref] [PubMed]
- Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update 2008;14:243-58. [Crossref] [PubMed]
- Calogero AE, Condorelli RA, Russo GI, et al. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed Res Int 2017;2017:4650182 [Crossref] [PubMed]
- Showell MG, Mackenzie-Proctor R, Brown J, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev 2014;CD007411 [PubMed]
Cite this article as: Le Saint C, Kadoch IJ, Bissonnette F, Choi J, Zini J, San Gabriel M, Zini A. Beneficial effect of antioxidant therapy on sperm DNA integrity is not associated with a similar effect on sperm chromatin integrity. AME Med J 2019;4:31.

