
Managing extragonadal germ cell tumors in male adults
Introduction
Germ cell tumors (GCTs) most commonly arise in the gonads with an incidence of 9,000 new cases yearly in the US, while extragonadal GCT (EGCTs) occur in the midline of the body represent around 5% of adult GCTs (1,2). EGCTs have a similar histology to testicular GCTs as both share a common chromosomal abnormality with gain of isochromosome 12p. However, their biology, prognosis and response to similar treatments are different, especially in primary mediastinal non-seminomatous GCT (PMNSGCT) (3). Even though males can develop EGCTs as neonatal, prepubertal, and adults, more than 90% of EGCTs occur in adult men between the ages of 20 and 35 years, and this category of patients will be the focus of this review (4). The most common primary site is the anterior mediastinum accounting for 50–60% of all EGCTs, followed by the retroperitoneum, with rare occurrence in the sacrococcygeal region and pineal gland. There is also a biological association between males with Klinefelter syndrome (KS) having an increased risk for GCTs, particularly PMNSGCT (5-7) with two large case series reporting an association in up to 20% of patients with KS (8,9). Furthermore, the risk of developing a GCT among males with KS was estimated to be 1:4,000; in comparison to males without KS, the risk was 19-fold higher (6). Interestingly, among those with primary mediastinal non-seminomatous GCT (PMNSGCTs), approximately one-third had KS. Therefore, screening has been proposed for KS among pediatric or adolescent males presenting with mediastinal GCTs (6).
EGCTs are some of the most thought-provoking tumors in clinical oncology as their pathogenesis has not been clearly elucidated with two competing hypotheses for tumorigenesis being proposed with limited data to definitively support either. The first, states that transformed germ cells in the testes undergo reverse migration during embryonal development, along the urogenital and gonadal ridges. This is supported by the available molecular data showing the shared cell of origin, while the opposing clinical and biological behaviors of these two tumors refute that hypothesis (10-12). The second hypothesis states that due to an abnormality in the primordial germ cell itself, EGCTs are derived from primordial germ cells that fail to complete the normal migration along the urogenital ridge to the gonadal ridges during embryonal development (13).
This focused review discusses the diagnosis and management of adult males with primary mediastinal and retroperitoneal GCTs.
Incidence, clinical presentation and diagnosis
Bokemeyer et al. described the clinical presentation of the largest cohort of patients with mediastinal and retroperitoneal EGCTs (14). In 635 consecutive patients treated at 11 centers, 341 patients (54%) had primary mediastinal EGCT, and 283 patients (45%) had retroperitoneal EGCT. Nonseminomatous germ cell tumor (NSGCT) histology was predominant with 524 patients (83%), and 104 patients (16%) having a seminomatous histology (14).
Chief presenting symptoms for PMNSGCTs were dyspnea (25%), chest pain (23%) and cough (17%), followed by fever (13%), weight loss (11%), vena cava occlusion syndrome and fatigue/weakness (6%). A very small number of patients presented with pathological cervical nodes (2%), hemoptysis, hoarseness or dysphagia (1% each) (14). On the other hand, in patients with retroperitoneal GCT, the most common presenting symptoms were abdominal (29%) and back pain (14%), followed by weight loss (9%), thrombosis (9%) and fever (8%). Other less frequent symptoms were palpable abdominal tumor (6%), cervical nodes (4%), scrotal edema (5%) and gynecomastia (3%) (14).
In the evaluation of a new bulky anterior mediastinal mass in a young male it is very important to investigate for mediastinal EGCTs. In the majority of patients (93%) the initial diagnosis can be established through the combination of computed scans, sonograms and tumor markers, with only a minority requiring tumor resection (2%) or biopsy (5%) as the initial diagnostic procedure (14). Nevertheless, imaging alone is not sufficient to complete the diagnostic workup and can minimally guide the histological diagnosis. Seminomas present as a homogeneous mass that is mildly enhancing, while a combination of calcification, fat, fluid, and soft tissue in an anterior mediastinal mass can be diagnostic of teratoma (15,16). However, the radiologic findings of other NSGCTs are often nonspecific, and tissue diagnosis is almost always needed. This was noted in the Bokemeyer et al. analysis where only 65 patients were classified without tissue confirmation based on the elevated tumor markers, while the remaining 570 patients required tissue confirmation to make the histopathological diagnosis (14). A marked difference from gonadal NSGCT, is the predominance of yolk sac tumors as the most common mediastinal NSGCT (60%), followed by mixed tumors (18%), choriocarcinoma (12%) and embryonal carcinoma (9%) (17).
Secondary malignancies
Interestingly around 10% of patients with PMNSGCT have been found to be associated with malignant hematological malignancies, specifically with acute megakaryoblastic leukemia or myelodysplasia (MDS) (18,19). This is unique to PMNSGCT, as these findings are not noted among those patients with testicular or retroperitoneal germ cell cancer treated with identical chemotherapy. These hematological malignancies are not therapy-related, as confirmed by clinical and cytogenetic studies that do not show the commonly associated mutations with therapy-related MDS or acute myeloid leukemia (AML). Furthermore, the defining karyotypic abnormality of germ cell cancer, isochromosome 12p, is found in the leukemic blasts of these patients (20). The median time between the diagnosis of PMNSGCT and secondary blood-related dyscrasias is estimated around 5 to 6 months. Moreover, the prognosis is poor with a shortened estimated median overall survival (18).
As for men with extragonadal GCTs, the risk of developing testicular invasive GCTs is highest in those with retroperitoneal rather than primary mediastinal disease. Hartmann et al. report in their meta-analysis, the estimated 10-year cumulative risk of metachronous testicular cancer after diagnosis of EGCT to be 10.3% (95% CI, 4.9% to 15.6%) (21). This cumulative risk was observed to be higher among patients with nonseminomatous EGCTs at 14.3%; 95% CI, 6.7% to 21.9%) and retroperitoneal EGCTs 14.2% (95% CI, 5.6% to 22.8%) in comparison to those with seminomatous EGCTs (1.4%; 95% CI, 0.0% to 4.2%) and mediastinal EGCTs (6.2%; 95% CI, 0.1% to 12.2%) (21). The risk of developing secondary non-germ cell solid tumors is not significantly increased with a reported incidence of 2% and a median follow-up time of close to 5 years (18).
Treatment approach
PMNSGCTs and retroperitoneal NSGCTs have a lower response to treatment than seminomatous EGCTs. The overall response rate for identical chemotherapy for stage matched patients with-in the two histological groups is 64% (95% CI, 58% to 70%) for PMNSGCT/NSGCTs and 68% (95% CI, 62% to 75%) for SGCTs while the 5-year OS is 45% and 62% respectively (14). Around 50% of patients with PMNSGCT will present with metastatic disease at the time of diagnosis, with 16% having metastatic disease in at least two organs or more (14). Therefore, we will begin by summarizing our approach to the treatment of patients with PMNSGCT, as shown in Figure 1.
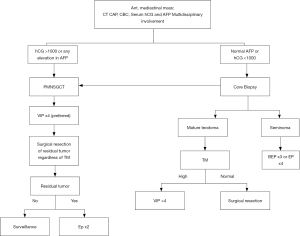
PMNSGCT
As discussed above, the diagnosis is first confirmed by: (I) a significant elevation in tumor markers, specifically human chorionic gonadotrophin (hCG) >1,000 U/L; (II) any elevation in α-fetoprotein (AFP); (III) in patients with normal tumor markers or with elevation in hCG only, a biopsy establishes the diagnosis. A multidisciplinary team approach should be considered to discuss and develop the team’s treatment plan as this leads to improved outcomes (22). All PMNSGCT patients are treated as high-risk GCTs and we recommend treatment with four courses of platinum-based triple chemotherapy, avoiding dose reduction, treatment delays and the use of Bleomycin. We recommend using etoposide (VP-16), ifosfamide and cisplatin (VIPx4) as the standard chemotherapy for PMNSGCT followed by surgical resection. The use of bleomycin in this specific population specifically is deferred in first line setting with the goal of mitigating severe respiratory complications if thoracic resection is planned post chemotherapy. For this reason, we would not encourage the use of bleomycin, etoposide and cisplatin (BEP) as frontline systemic therapy. Similarly, we do not recommend first line treatment with high-dose chemotherapy and autologous stem cell transplant, as no prospective study has till date evaluated its benefit.
Role of post-chemotherapy surgical resection for PMNSGCT
One of the important clinical pearls that clinicians should be aware of when treating patients with PMNSGCT, is that surgical resection of any residual disease is a crucial part of the management of all of these patients. In the absence of effective salvage chemotherapy and knowing that 30% to 46% of patient will have residual viable tumor after chemotherapy, regardless of the level of tumor markers, all patients should receive post-chemotherapy surgical resection (23,24). This is different from the management of patients with testicular germ cell tumor, where surgical resection is reserved to those with only normalized markers (25,26). Surgical resection has three main roles, first to assess tumor response, second to resect chemotherapy-resistant disease, and lastly to guide the use of adjunctive chemotherapy post-surgical resection.
After complete resection of the tumor, for patients with residual viable germ cell component in the surgical specimen, we recommend the patient receives two additional cycles of etoposide with cisplatin (EP) (27). As for patients with only necrosis or teratoma in the surgical specimen we recommend active surveillance with history, physical exam, hCG, AFP and computed tomography (CT) scans every 2 months for the first year, every 3 months for the second year, and thereafter every 6 months for years 3 to 5 (27). There is no proven role for PET-CT imaging for surveillance.
Management of relapsed PMNSGCT
FDG PET-CT imaging is useful for the detection recurrent disease in the setting of elevated markers with normal CT imaging and in discerning viable tumor in the residual mass after chemotherapy (28).
Patients who relapse after initial cisplatin-based chemotherapy have dismal outcomes with only 7–10% of men, who had a primary mediastinal NSGCT, experiencing a durable remission with subsequent standard dose chemotherapy (14,18,29). The role of surgical resection in the setting of relapsed disease with increasing tumor markers has been very controversial. Nevertheless, it should be considered if the surgery is performed by experienced thoracic surgeons. This was shown in a case series reporting on the Indiana University experience comprised of 35 patients with relapsed PMNSGCTs and rising tumor markers treated with surgical resection alone. A 20% of disease-free survival was observed post resection, with a median follow-up of 64 months (range, 25–220 months) (30). If surgery is not a possibility, we would highly encourage enrollment in a clinical trial for these patients testing new treatment strategies. If this is not possible, we would recommend the evaluation for high-dose chemotherapy and autologous stem cell transplant in the second line (31). One study of 59 patients evaluated 37 patients with retroperitoneal and 22 with mediastinal NSGCTs. Their findings reported minimally better outcomes with 15 (41%) of the patients with retroperitoneal primary tumors were alive and continuously disease-free at the time of the study publication, compared to only three (14%) of the mediastinal NSGCT patients (32). Furthermore, the retrospective series by Adra et al. looked at 364 consecutive patients with GCT who progressed after cisplatin-based combination chemotherapy and were subsequently treated with high-dose chemotherapy followed by autologous stem-cell transplantation at Indiana University. Of these, 20 patients had PMNSGCT, with a two-year PFS rate of 23% (95% CI, 7% to 43%) versus 63% (95% CI, 57% to 68%; P<0.001) for patients with testicular/retroperitoneal primary tumor sites (31). Knowing that these results are discouraging, other options need to be explored in this challenging population. We would consider assessing if these tumors harbor a high tumor mutational burden or high levels of microsatellite instability (MSI-H), which may predict benefits from immune checkpoint inhibitor immunotherapy (33). The US Food and Drug Administration (FDA) approved pembrolizumab for treatment of a variety of advanced solid tumors that are MSI-H or have deficient mismatch repair, and which progressed following prior treatment. Accordingly, pembrolizumab could be considered as an option in patients demonstrating MSI-H, the current information regarding immune checkpoint inhibitors remains limited and disappointing for patients with refractory testicular GCTs (26,34,35). First, a case series of seven patients treated with nivolumab or pembrolizumab for refractory GCTs, reported two long-term responses, both in patients whose tumors were highly positive for programmed cell death ligand 1 (PD-L1) staining (34). Unfortunately, a phase 2 trial evaluating treatment by pembrolizumab in 12 men unselected for PD-L1 expression with relapsed GCTs, reported no observed partial responses (35).
Primary mediastinal teratoma and seminoma
Mediastinal mature teratoma, the most common GCT in the mediastinum, is treated with surgical resection. In fact, the only role for chemotherapy is in the situation when the presentation is associated with elevated tumor markers (Figure 1). It is very important for clinicians to always consider the rare presentation of growing mediastinal mass causing cardiopulmonary deterioration, precluding the continuation of chemotherapy, named ‘growing teratoma syndrome’. The rapid recognition of this syndrome, cessation of chemotherapy, and immediate surgical intervention, are crucial to cure these patients (36).
We recommend that patients with seminomatous EGCTs should be treated according to their prognostic international germ cell consensus classification (IGCCCG). This is in accordance with the findings from a meta-analysis of 104 patients with primary mediastinal and retroperitoneal extragonadal seminomas showing similar clinical outcomes and prognosis to primary gonadal seminomas, with an estimated 5-year survival of 88% (37). Therefore, patients with good prognosis mediastinal seminoma should be treated with three cycles of BEP, while patients with intermediate prognosis would require four cycles of BEP (38).
It is also very important to highlight that survivors should be regularly monitored for the multiple potential late toxicities of GCT therapy such as cardiovascular disease, metabolic syndrome, hypogonadism, ototoxicity, osteoporosis, retinal toxicity, pulmonary toxicity, and depression (26).
Primary retroperitoneal GCTs
It is important to differentiate between retroperitoneal GCTs that are metastases from primary testicular GCTs and primary retroperitoneal GCT (PRGCTs). These patients should undergo testis ultrasound to rule out a testis primary, but a biopsy is not to be performed if there is no evidence of testicular tumor on ultrasound.
PRGCTs represent 30–40% of EGCTs, and the international consensus classification makes no distinction between PRGCTs and testis primary. Therefore, therapy is based upon published risk criteria for good, metastatic testicular GCT (27,39). Surgical resection is also an integral part of the management of retroperitoneal nonseminomatous GCTs with residual mass after chemotherapy. Indeed, from patients who underwent surgical resection for retroperitoneal residues, 25% had viable tumor, 16% had mature teratomas, and 59% had necrotic tissue (40).
Conclusions
EGCTs are rare, unique and more aggressive than testicular germ cell cancers. PMNSGCT has the worst survival, with a high rate of relapse post cisplatin based front line therapy. Therefore, surgical resection is an essential component of the management of these tumors when feasible. Also, these patients have a predisposition to develop secondary blood related malignancies. A large number of patients with PMNSGCT will die from their disease. Unfortunately, limited progress has been made in understanding the molecular and immune component of this disease to inform treatment development for patients with relapsed PMNSGCT (41,42). Further research is needed to guide clinical trials that will improve the outcome of EGCTs.
Acknowledgments
Funding: Dr. Chahoud is supported by the ASCO Conquer Cancer Foundation Young Investigator Award.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Philippe E. Spiess) for the series “Rare Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.01.01/coif). The series “Rare Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stang A, Trabert B, Wentzensen N, et al. Gonadal and extragonadal germ cell tumours in the United States, 1973-2007. Int J Androl 2012;35:616-25. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Schneider DT, Schuster AE, Fritsch MK, et al. Genetic analysis of mediastinal nonseminomatous germ cell tumors in children and adolescents. Genes Chromosomes Cancer 2002;34:115-25. [Crossref] [PubMed]
- Rusner C, Trabert B, Katalinic A, et al. Incidence patterns and trends of malignant gonadal and extragonadal germ cell tumors in Germany, 1998-2008. Cancer Epidemiol 2013;37:370-3. [Crossref] [PubMed]
- Völkl TMK, Langer T, Aigner T, et al. Klinefelter syndrome and mediastinal germ cell tumors. Am J Med Genet A 2006;140:471-81. [Crossref] [PubMed]
- Williams LA, Pankratz N, Lane J, et al. Klinefelter syndrome in males with germ cell tumors: A report from the Children’s Oncology Group. Cancer 2018;124:3900-8. [Crossref] [PubMed]
- Aguirre D, Nieto K, Lazos M, et al. Extragonadal germ cell tumors are often associated with Klinefelter syndrome. Hum Pathol 2006;37:477-80. [Crossref] [PubMed]
- Dexeus FH, Logothetis CJ, Chong C, et al. Genetic abnormalities in men with germ cell tumors. J Urol 1988;140:80-4. [Crossref] [PubMed]
- Nichols CR, Heerema NA, Palmer C, et al. Klinefelter’s syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol 1987;5:1290-4. [Crossref] [PubMed]
- Chaganti RS, Houldsworth J. Genetics and biology of adult human male germ cell tumors. Cancer Res 2000;60:1475-82. [PubMed]
- Hailemariam S, Engeler DS, Bannwart F, et al. Primary mediastinal germ cell tumor with intratubular germ cell neoplasia of the testis--further support for germ cell origin of these tumors: a case report. Cancer 1997;79:1031-6. [Crossref] [PubMed]
- Chaganti RS, Rodriguez E, Mathew S. Origin of adult male mediastinal germ-cell tumours. Lancet 1994;343:1130-2. [Crossref] [PubMed]
- Glenn OA, Barkovich AJ. Intracranial germ cell tumors: A comprehensive review of proposed Embryologic Derivation. Pediatr Neurosurg 1996;24:242-51. [Crossref] [PubMed]
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: Results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
- Suzuki M, Takashima T, Itoh H, et al. Computed tomography of mediastinal teratomas. J Comput Assist Tomogr 1983;7:74-6. [Crossref] [PubMed]
- Takahashi K, Al-Janabi NJ. Computed tomography and magnetic resonance imaging of mediastinal tumors. J Magn Reson Imaging 2010;32:1325-39. [Crossref] [PubMed]
- Moran CA, Suster S, Koss MN. Primary germ cell tumors of the mediastinum: III. Yolk sac tumor, embryonal carcinoma, choriocarcinoma, and combined nonteratomatous germ cell tumors of the mediastinum--a clinicopathologic and immunohistochemical study of 64 cases. Cancer 1997;80:699-707. [Crossref] [PubMed]
- Hartmann JT, Nichols CR, Droz JP, et al. Hematologic disorders associated with primary mediastinal nonseminomatous germ cell tumors. J Natl Cancer Inst 2000;92:54-61. [Crossref] [PubMed]
- Nichols CR, Roth BJ, Heerema N, et al. Hematologic neoplasia associated with primary mediastinal germ-cell tumors. N Engl J Med 1990;322:1425-9. [Crossref] [PubMed]
- Ladanyi M, Samaniego F, Reuter VE, et al. Cytogenetic and immunohistochemical evidence for the germ cell origin of a subset of acute leukemias associated with mediastinal germ cell tumors. J Natl Cancer Inst 1990;82:221-7. [Crossref] [PubMed]
- Hartmann JT, Fossa SD, Nichols CR, et al. Incidence of metachronous testicular cancer in patients with extragonadal germ cell tumors. J Natl Cancer Inst 2001;93:1733-8. [Crossref] [PubMed]
- Albany C, Adra N, Snavely AC, et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol 2018;29:341-6. [Crossref] [PubMed]
- Kesler KA, Rieger KM, Ganjoo KN, et al. Primary mediastinal nonseminomatous germ cell tumors: the influence of postchemotherapy pathology on long-term survival after surgery. J Thorac Cardiovasc Surg 1999;118:692-700. [Crossref] [PubMed]
- Vuky J, Bains M, Bacik J, et al. Role of postchemotherapy adjunctive surgery in the management of patients with nonseminoma arising front the mediastinum. J Clin Oncol 2001;19:682-8. [Crossref] [PubMed]
- Einhorn L. MTE 23.02 Mediastinal Germ Cell Tumor. J Thorac Oncol 2017;12:S1652-3. [Crossref]
- Chahoud J, Zhang M, Shah A, et al. Managing seminomatous and nonseminomatous germ cell tumors. Curr Opin Oncol 2018;30:181-8. [PubMed]
- Albany C, Einhorn LH. Extragonadal germ cell tumors: clinical presentation and management. Curr Opin Oncol 2013;25:261-5. [Crossref] [PubMed]
- Shinagare AB, Jagannathan JP, Ramaiya NH, et al. Adult extragonadal germ cell tumors. AJR Am J Roentgenol 2010;195:W274-80. [Crossref] [PubMed]
- Broun ER, Nichols CR, Kneebone P, et al. Long-term outcome of patients with relapsed and refractory germ cell tumors treated with high-dose chemotherapy and autologous bone marrow rescue. Ann Intern Med 1992;117:124-8. [Crossref] [PubMed]
- Radaideh SM, Cook VC, Kesler KA, et al. Outcome following resection for patients with primary mediastinal nonseminomatous germ-cell tumors and rising serum tumor markers post-chemotherapy. Ann Oncol 2010;21:804-7. [Crossref] [PubMed]
- Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana university experience. J Clin Oncol 2017;35:1096-102. [Crossref] [PubMed]
- De Giorgi U, Demirer T, Wandt H, et al. Second-line high-dose chemotherapy in patients with mediastinal and retroperitoneal primary non-seminomatous germ cell tumors: The EBMT experience. Ann Oncol 2005;16:146-51. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Zschäbitz S, Lasitschka F, Hadaschik B, et al. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer 2017;76:1-7. [Crossref] [PubMed]
- Adra N, Einhorn LH, Althouse SK, et al. Phase II trial of pembrolizumab in patients with platinum refractory germ-cell tumors: a Hoosier Cancer Research Network Study GU14-206. Ann Oncol 2018;29:209-14. [Crossref] [PubMed]
- Kesler KA, Patel JB, Kruter LE, et al. The “growing teratoma syndrome” in primary mediastinal nonseminomatous germ cell tumors: criteria based on current practice. J Thorac Cardiovasc Surg 2012;144:438-43. [Crossref] [PubMed]
- Bokemeyer C, Droz JP, Horwich A, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer 2001;91:1394-401. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®) Testicular Cancer, 2019.
- van Dijk MR, Steyerberg EW, Stenning SP, et al. Survival of patients with nonseminomatous germ cell cancer: a review of the IGCC classification by Cox regression and recursive partitioning. Br J Cancer 2004;90:1176-83. [Crossref] [PubMed]
- Schmoll HJ. Extragonadal germ cell tumors. Ann Oncol 2002;13:265-72. [Crossref] [PubMed]
- Kollmannsberger C, Nappi L, Nichols C. Novel treatment options for refractory Germ Cell Tumours: Back to the bench! Ann Oncol 2018;29:21-2. [Crossref] [PubMed]
- Oing C, Bokemeyer C. Biological basis and early clinical results of immunotherapy for cisplatin-resistant germ cell cancer. Curr Opin Urol 2018;28:479-84. [Crossref] [PubMed]
Cite this article as: Chahoud J, Kohli M. Managing extragonadal germ cell tumors in male adults. AME Med J 2020;5:8.

