
Lymph node dissection during radical cystectomy: indications, extension and impact on survival outcomes
Bladder cancer (BC) remains one of the most common cancer worldwide. The reference option of treatment for muscle invasive bladder cancer (MIBC) and for recurrent non-muscle invasive disease is radical cystectomy (RC) and pelvic lymph node dissection (PLND) with urinary diversion (UD). PLND is a crucial step in BC management even if its clinical significance, as a staging instrument and/or a therapeutic procedure, is still controversial (1).
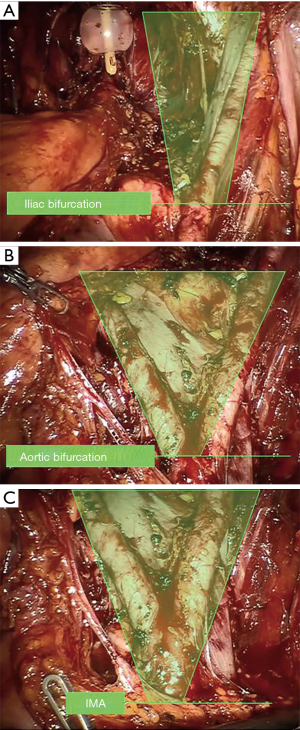
Nowadays, staging role of PLND is widely accepted, therefore the debate has moved on its extension. Standard PLND (s-PLND) includes removal of internal iliac, obturator fossa and external iliac nodes, describing the iliac bifurcation as the upper boundary, the ureter and the genitofemoral nerves as medial and lateral borders and circumflex iliac vein, lacunar ligament and Cloquet’s lymph node as lower limit (2-5). The term “extended” PLND (e-PLND) refers to template including lymph nodes up to aortic bifurcation, thus lymph nodes of the standard template plus the presacral and common iliac nodes. Finally, super-extended PLND (se-PLND) includes also nodes above the aortic bifurcation, up to the origin of the inferior mesenteric artery (6) (Figure 1).
Since 2009, the TNM staging system (1) considered pN3 stage as positive metastasis in a common iliac lymph node, therefore including common iliac nodes in the template is considered as mandatory step to provide proper staging. Bruins et al. in a systematic review suggested that in terms of oncologic outcomes, any extent of LND is more effective than non-LND. Notwithstanding, if limited PLND (incomplete removal of lymph nodes included in the standard template) should be not considered as an option, there is still an ongoing debate about the effectiveness of s-PLND compared with e-PLND or se-PLND. There are some evidences from retrospective studies about the absence of oncologic benefits for se-PLND versus e-PLND, with increased operative time and non-significant increase in postoperative morbidity (7).
Recently, Gschwend et al. conducted a prospective randomized trial comparing se-PLND vs. s-PLND (8). Median number of retrieved lymph nodes was 19 (IQR: 12–26) vs. 31 (IQR: 22–47) in se-PLND. The 5-year recurrence free survival (RFS) was 64.6% and 59.2% in se-PLND and s-PLND groups, respectively (P=0.36). The 5-year cancer specific survival (CSS) rate was 75.9% and 64.5% in se-PLND and s-PLND groups, respectively (P=0.10). The 5-year overall survival (OS) rate was 58.9% and 49.7% in se-PLND and s-PLND groups, respectively (P=0.12). Therefore, the study failed to show a statistically significant oncologic advantage of se-PLND over s-PLND. Perioperative outcomes were comparable between groups. Overall, mortality and short- and long-term severe complications (Clavien grade ≥3) did not differ between cohorts. Exclusively, the study described in se-PLND group a higher rate of lymphoceles (8.6%), which required postoperative treatments, compared to s-PLND group (3.4%) (P=0.04). In summary, the study was intended to prove an absolute improvement of 15% in 5-year RFS by extended LND based on retrospective data, but it failed to reveal an oncologic benefit in terms of RFS, CSS and OS. However, this keenly awaited prospective randomised trial has opened an important debate and several additional comments were reported. Gakis (9) underlined critical aspects related to the nattiness of lymph node sampling method. In fact, the study design was referred to Leissner et al. (10) but in this study the mean lymph node yield was almost 30% higher for e-PLND. Moreover, Soria et al. (11) showed some confounders that could have contributed to results obtained. First, PLND templates described s-PLND versus se-PLND rather that l-PLND versus e-PLND. Secondly, in the study were reported a relatively high rate of patients (14%) suffering from a high-grade pT1 non-muscle-invasive disease that may have limited the strength of results. Therefore, the extent of LND remains debatable, and literature still needs more prospective randomised trials to assess the proper extension of PLND in order to clearly provide guidance in clinical practice. Last but not least, there was a survival benefit in the se-PLND cohort, although not significant. This is likely to be based on underpowered enrolment and study limitations.
In previous retrospective reports, Simone et al. (12) consistent with the SR of Bruins et al. (7), supported that e-PLND has both staging and therapeutic role reporting better oncologic outcomes of patients underwent e-PLND in comparison with s-PLND. They showed that patients underwent an e-PLND had a significant improvement of disease-free survival (DFS) (HR 1.96, P<0.001) and CSS (HR 1.76, P<0.001) probabilities compared to s-PLND, describing a therapeutic result of extending PLND from the iliac bifurcation up to the aortic bifurcation. Particularly, at 1-year, 3-year and 5-year of follow-up, DFS probabilities in s-PLND group were 71.2%, 49.4% and 42.6%, respectively; while, at the same time, in patients underwent e-PLND were observed higher DFS probabilities (86%, 68.6% and 63.1%; P<0.001). Similarly, 1-year, 3-year and 5-year CSS probabilities were 87.7%, 62% and 50.9% in s-PLND cohort, and 93.5%, 78.5% and 68.8% in the e-PLND group, respectively (P<0.001). Moreover, they described that the number of nodes removed (LN-c) was able to predict, at univariable analysis, DFS (P=0.003) and CSS (P=0.018) probabilities, even if this predictive role was not significant at multivariable analysis, suggesting the crucial role of establishing the anatomical template of PLND. Consequently, LN-c alone has no role in predicting survival outcomes, but it is to be considered as a consequence of PLND extent, therefore secondary to the anatomical template of PLND performed. Several studies underlined the major predictive role of LN-c rather than pN stage in predicting CSS, despite the lack of strong evidences related to the minimum of LN-c. In 2003, Herr (13) and Stein et al. (14) had already described superiority of LN density (LN-d) over pN stage in predicting oncologic outcomes, according to the capacity of describing quality and extent of LND, that is not possible to assess only with pN stage. Karl et al. (15) recommended removal of at least 20 lymph nodes to properly stage BC, and Quek et al. (16) defined a variable cut-off point of LN-d ranging from 4% to 25%. Simone et al. (17) identified as optimal cut-off a LN-d between 12% and 30%. They reported LN-d cut-off points (<12%, 12–30% and >30%) were the only significant independent factors able to predict CSS probability (12–30% vs. <12%: HR 1.51, P=0.047; >30% vs. <12%: HR 2.89, P<0.001), and median CSS of patients were also significantly different for each LN-d cut-off point: 71, 24 and 11 month, respectively (P<0.001).
Actually, despite the ongoing debate about the proper extent of PLND, there are specific conditions where limiting the extent of PLND is a common practice. For instance, in case of sex-sparing RC, when any effort should be performed to avoid unintentional injury of sympathetic nerves. The sympathetic superior hypogastric plexus (the presacral nerve) lies below the aortic bifurcation in the outer stratum of the extraperitoneal connective tissue over L5 and the sacral promontory, then with right and left hypogastric nerves descend on the pelvic sidewall medial to the internal iliac artery. They are connected by the pelvic splanchnic nerves before proceeding to their respective plexuses, the right and left inferior hypogastric plexuses near the bladder base, the prostate, and the seminal vesicles. Therefore, it is crucial to avoid the extension of PLND to the presacral area and medially to the internal iliac artery, since only preserving these anatomical structures it is possible to preserve autonomic nerves during PLND, achieving the significant improvement of functional outcomes during a sex-sparing approach.
Over the years, as a result of constantly increase of life’s expectancy and a general aging of the population, it has been reported a progressive increase in the median age of patients undergoing RC, which raised from a median of 62 years in 1990 to 70 years in 2012 (18). In this scenario, RC was increasingly adopted even in elderly patients. Bream et al. (19) reported data from National Cancer Database of patients older than 75 years, affected by non-metastatic MIBC, who underwent, between 2003 and 2012, RC, chemoradiation therapy or alternative nonstandard treatments. Between the study period, the rate of patients undergoing RC has almost doubled (14% to 24%, from 2003 to 2012, respectively; P<0.01), and they had better survival outcomes compared with patients treated with alternative nonstandard treatments. At the same time, Horovitz et al. (20) underlined that, even if RC is an established complex surgery associated with high risk of perioperative, short and long term complications, it has be considered as a feasible option also in the octogenarian population, affirming that RC should not precluded only for age.
In fact, they reported that for patients suffering for BCa, the leading cause of death is disease progression, independently from any other variables such as: age, pT and/or pN stage (18). Despite evidences supporting that age alone is not an absolute contraindication for RC, there are few data in literature regarding the actual role of PLND in elderly population. Grabbert et al. (21) reported that PLND did not have a benefit on survival outcomes. Univariable and multivariable analysis failed to show a statistically significant improvement of PLND on CSS (P=0.606), OS (P=0.979) and PFS (P=0.883) in elderly population. Moreover, they described a significant longer operative time (PLND 215 min vs. non-PLND 182 min; P=0.003) without significant differences in post-operative complications (Clavien ≥3 in 27% vs. 21%, in PLND and no PLND group, respectively; P=0.563). Therefore, even if we need more series and prospective randomized trials, it should be reasonable to omit PLND in elderly patients during RC.
Nowadays, open RC and PLND is still considered the reference option of treatment, even if the widespread use of robotic surgery is progressively challenging this paradigm. However, data are immature to compare both approaches, therefore robot-assisted laparoscopic radical cystectomy (RARC) is still considered an investigational procedure. Several studies showed that robotic approach reduces blood loss and transfusions, and it has a shorter length of hospital stay compared to open radical cystectomy (ORC) (22,23). Despite these evidences, all the current randomized controlled trials (RCTs), including intermediate and long-term oncologic and functional outcomes, did not prove any significant difference (24-26).
Simone et al. (22) reported a propensity score matched analysis, to compare perioperative and mid-term oncologic outcomes of RARC with totally intracorporeal neobladder with open cohort, from a single centre series. In the open cohort, a higher incidence of perioperative complications was observed, most of which represented by blood transfusions. Comparable oncologic outcomes and also comparable PLND outcomes were observed; a mean LN-c of 33.4 (±12.3) and 30.7 (±14.1) for robotic and open approach (P=0.16) was reported, respectively.
Recently, we underlined the importance to follow the technological progress in robotic surgery field, in order to outclass the ancient concept of “replicating the open principles” (27). Actually, robotic surgery failed to show its superiority over open surgery, and several studies in literature reported comparable oncologic and functional outcomes between RARC and ORC, emphasizing non-inferiority of robotic surgery over the traditional and standardized open surgery. We are strongly convinced that this is the first step to establish the role of robotic surgery in the management of BC, and now the technological revolution will guide robotic surgery to affirm its definitive role. There is a growing interest in the use of near-infrared fluorescence technology that is able to enhance visualization of anatomical structures. In the setting of PLND for BC, Manny et al. (28) described a useful role of near-infrared fluorescence (NIRF) technology during RC. Through the cystoscopically injection of indocyanine green (ICG) into the lesion, it is possible to enhance tumor margins and to easily recognize sentinel lymph node. It has reported a 90% of rate to identify sentinel nodal drainage, and NIRF technology has proved to be a highly sensitive tool in the identification of a nodal involvement.
In summary, PLND is an essential step in BC management with an established staging role and a possible therapeutic role with impact on survival outcomes following RC. Concerning the proper extent of PLND, there is available Literature supporting the role of a template bases PLND, being the prognostic role of LN-c itself inconclusive and misleading. The minimum number of LN-c to assess a well performed PLND cannot be determined, while performing at least a s-PLND is nowadays no longer matter of debate. With regard to the role of robotic surgery in this setting, PLND during RARC provides comparable outcomes of those obtained with ORC. As observed for the development of NIRF imaging, the technological development is likely to provide increasing benefits in the field of robotic surgery that can be true “game changer” in this field.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Moschini) for the series “Bladder Cancer” published in AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.01.05/coif). The Series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Witjes JA, Bruins M, Cathomas R, et al. EAU Guidelines on muscle-invasive and metastatic bladder cancer. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Muscle-invasive-and-Metastatic-Bladder-Cancer-Guidelines-2016.pdf
- Holmer M, Bendahl PO, Davidsson T, et al. Extended lymph node dissection in patients with urothelial cell carcinoma of the bladder: Can it make a difference? World J Urol 2009;27:521-6. [Crossref] [PubMed]
- Poulsen AL. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol 1998;160:2015-9; discussion 2020.
- Jensen JB, Ulhøi BP, Jensen KME. Extended versus limited lymph node dissection in radical cystectomy: Impact on recurrence pattern and survival. Int J Urol 2012;19:39-47. [Crossref] [PubMed]
- Dhar NB, Klein EA, Reuther AM, et al. Outcome After Radical Cystectomy With Limited or Extended Pelvic Lymph Node Dissection. J Urol 2008;179:873-8; discussion 878. [Crossref] [PubMed]
- Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: A comparative study. J Urol 2011;186:1261-8. [Crossref] [PubMed]
- Bruins HM, Veskimae E, Hernandez V, et al. The impact of the extent of lymphadenectomy on oncologic outcomes in patients undergoing radical cystectomy for bladder cancer: A systematic review. Eur Urol 2014;66:1065-77. [Crossref] [PubMed]
- Gschwend JE, Heck MM, Lehmann J, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol 2019;75:604-11. [Crossref] [PubMed]
- Gakis G. Re: Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol 2019;76:126. [Crossref] [PubMed]
- Leissner J, Ghoneim MA, Abol-Enein H, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: Results of a prospective multicenter study. J Urol 2004;171:139-44. [Crossref] [PubMed]
- Soria F, Gontero P. Re: Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol 2019;76:408-9. [Crossref] [PubMed]
- Simone G, Papalia R, Ferriero M, et al. Stage-specific impact of extended versus standard pelvic lymph node dissection in radical cystectomy. Int J Urol 2013;20:390-7. [Crossref] [PubMed]
- Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003;169:943-5. [Crossref] [PubMed]
- Stein JP, Cai J, Groshen S, et al. Risk factors for patients with pelvic lymph node metastases following radical cystectomy with en bloc pelvic lymphadenectomy: The concept of lymph node density. J Urol 2003;170:35-41. [Crossref] [PubMed]
- Karl A, Carroll PR, Gschwend JE, et al. The Impact of Lymphadenectomy and Lymph Node Metastasis on the Outcomes of Radical Cystectomy for Bladder Cancer. Eur Urol 2009;55:826-35. [Crossref] [PubMed]
- Quek ML, Flanigan RC. The role of lymph node density in bladder cancer prognostication. World J Urol 2009;27:27-32. [Crossref] [PubMed]
- Simone G, Papalia R, Ferriero M, et al. Development and external validation of lymph node density cut-off points in prospective series of radical cystectomy and pelvic lymph node dissection. Int J Urol 2012;19:1068-74. [Crossref] [PubMed]
- Moschini M, Martini A, Zamboni S, et al. Evaluation of Cause of Death After Radical Cystectomy for Patients With Bladder Cancer: The Impact of Age at the Time of Surgery. Clin Genitourin Cancer 2019;17:e541-8. [Crossref] [PubMed]
- Bream MJ, Maurice MJ, Altschuler J, et al. Increased Use of Cystectomy in Patients 75 and Older: A Contemporary Analysis of Survival and Perioperative Outcomes From the National Cancer Database. Urology 2017;100:72-8. [Crossref] [PubMed]
- Horovitz D, Turker P, Bostrom PJ, et al. Does patient age affect survival after radical cystectomy? BJU Int 2012;110:E486-93. [Crossref] [PubMed]
- Grabbert M, Grimm T, Buchner A, et al. Risks and benefits of pelvic lymphadenectomy in octogenarians undergoing radical cystectomy due to urothelial carcinoma of the bladder. Int Urol Nephrol 2017;49:2137-42. [Crossref] [PubMed]
- Simone G, Tuderti G, Misuraca L, et al. Perioperative and mid-term oncologic outcomes of robotic assisted radical cystectomy with totally intracorporeal neobladder: Results of a propensity score matched comparison with open cohort from a single-centre series. Eur J Surg Oncol 2018;44:1432-8. [Crossref] [PubMed]
- Tyritzis SI, Collins JW, Wiklund NP. The current status of robot-assisted cystectomy. Indian J Urol 2018;34:101-9. [Crossref] [PubMed]
- Khan MS, Gan C, Ahmed K, et al. A single-centre early phase randomised controlled three-arm trial of open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur Urol 2016;69:613-21. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: A randomized clinical trial. Eur Urol 2015;67:1042-50. [Crossref] [PubMed]
- Messer JC, Punnen S, Fitzgerald J, et al. Health-related quality of life from a prospective randomised clinical trial of robot-assisted laparoscopic vs. open radical cystectomy. BJU Int 2014;114:896-902. [Crossref] [PubMed]
- Brassetti A, Mastroianni R, Simone G. Are we really seeking for equivalence?—The virtue of the robot is in technology. Transl Androl Urol 2019;8:S502-4. [Crossref] [PubMed]
- Manny TB, Hemal AK. Fluorescence-enhanced Robotic Radical Cystectomy Using Unconjugated Indocyanine Green for Pelvic Lymphangiography, Tumor Marking, and Mesenteric Angiography: The Initial Clinical Experience. Urology 2014;83:824-9. [Crossref] [PubMed]
Cite this article as: Mastroianni R, Tuderti G, Simone G. Lymph node dissection during radical cystectomy: indications, extension and impact on survival outcomes. AME Med J 2020;5:22.

