
Multiple and total arterial coronary artery bypass grafting
Introduction
In 1968, the first artery-to-coronary anastomosis involving the left internal thoracic artery (LITA) and the left anterior descending coronary artery (LAD) was performed by Dr. Green (1). Since then, the use of this arterial conduit has proven to provide a survival benefit and a reduced risk of repeat revascularization, thereby establishing its status as the standard intervention during coronary artery bypass grafting (CABG) in patients with LAD disease (2-5). The idea of adding other arterial conduits and the conceptualization of multiple (MAG) and total arterial grafting (TAG) came intuitively after the acknowledgment of the benefit associated with the left internal thoracic artery (LITA) coupled with a worse patency rate (50% at 10 years) and outcomes of saphenous vein graft (SVG) (6). The use of more than 1 arterial graft has been reported to overcome long-term recurrence of myocardial ischemia and patency failure.
Multiple arterial grafting
Bilateral internal thoracic arteries
The first randomized clinical trial (RCT) compared single internal thoracic artery (SITA) with bilateral internal thoracic arteries (BITA) in 162 patients and 81 patients in each arm. Authors concluded that early and 5-year (overall mortality and cardiac event-free survival) outcomes of the 2 groups were equal. Unfortunately, the limited number of patients did not allow for the clinical differences in the 2 arms to be evaluated (7).
In 2009, the results of the Stand-in-Y mammary study (8) were published. This was a randomized controlled trial (RCT) conducted in Italy consisting of 4 arms for a total of 815 patients, and included 2 BITA groups with 2 different methods, 1 SITA plus radial artery (RA) and 1 SITA plus SVG. After a brief follow-up of 2 years, no differences in overall survival were found between the groups, but patients randomized to receive 2 arterial grafts showed a benefit in cardiac event-free survival.
The Arterial Revascularization Trial (ART) (9) was a multicenter, randomized, unblinded trial which enrolled 3102 patients that were assigned to receive either BITA or SITA. Notably, the number of patients to be enrolled was decided in order to detect an absolute 5% reduction in overall survival at 10 years (10). In the intention-to-treat analysis at 10 years, neither the primary outcome, overall survival, nor the event-free survival showed any difference among the 2 groups (HR 0.96; 95% CI, 0.82–1.12). It is worth mentioning that the trial was burdened by a high crossover rate between the 2 arms so that 14% of patients assigned to the BITA group actually received SITA. Also, in the SITA arm 21% of patients received a second arterial graft (RA). Therefore, when an as-treated analysis was carried out, a survival benefit and a reduction in major adverse cardiovascular events were reported in the MAG group (LITA-RA or BITA) versus the single arterial graft group. Several reasons can explain this discrepancy. First of all, the high crossover rate and the frequent use of RA could have both substantially narrowed the difference in clinical outcomes between the 2 groups. In addition, power calculation for the study was based on literature sources dating back to the 1970s and therefore did not take into account the improvements in care of recent years. Additionally, the as-treated analysis remains an observational comparison, and thus selection bias and other confounders might have impacted the results (11).
Contrasting the results of the biggest randomized trial comparing BITA and SITA, the evidence from observational studies, pooled together in systematic reviews and meta-analysis, have highlighted a positive survival advantage for BITA (12-18). Gaudino et al. attempted to address the contradiction between observational studies and randomized trials with a meta-analysis and suggested that the superiority of BITA seen in observational studies is related to unmeasured confounders rather than an actual biological advantage (19).
The adoption of BITA in daily practice is still limited. In 2016, BITA was used in 12–15% and 5.5% of coronary revascularizations in Europe and the USA respectively (20). Current guidelines recommend the use of BITA in patients with no higher risk of sternal wound infection (class IIa) (4), and concerns regarding the use of BITA are most noticeably limiting its valuable use in surgical practice. In particular, the risk of deep sternal wound infection (DSWI) is highly feared in patients with poorly controlled diabetes, chronic pulmonary obstructive disease, previous mediastinal irradiation, and obesity. Sternal complications are mostly due to the reduction in vascularization of tissue surrounding the thoracic artery. Hence, the use of a skeletonized technique to harvest BITA has been shown to reduce the risk of sternal complications compared to pedicled SITA harvesting (21).
Radial artery
According to current guidelines (4), the use of RA to perform MAG is a Class I recommendation. In order to avoid a major problem regarding competitive flow, only target coronary vessels with a stenosis of at least 70% and ideally of 90% of the vessel lumen should be considered to be grafted with RA. An aggregate meta-analysis of 6 RCTs that compared RA with SVG showed a decreasing trend in repeat revascularization, myocardial infarction, and cardiac death, in favor of RA (22). Similarly, a pooled individual-patient analysis of 6 RCTs, comprising 1,036 patients, showed that RA grafting leads to a reduction of the composite outcome of death, myocardial infarction, and repeat revascularization. Individually, myocardial infarction and repeat revascularization rates were lower in patients receiving RA than those receiving SVG, whereas mortality did not show a significant difference. As expected in an angiographic trial, the difference in the composite outcome was primarily driven by repeat revascularization and was mainly due to the higher patency rate of the RA graft (23).
Notably, only the Radial Artery Patency and Clinical Outcomes (RAPCO) trial compared RITA and RA in relation to MAG. At the 96th Annual Meeting of the American Association of Thoracic Surgery, the 10-year results of this trial were presented and showed that the use of RA was associated with a benefit in overall survival, whereas the patency rate was similar (24).
The evidence from observational studies comparing RA and SVG was pooled in a recent meta-analysis (25) in which authors found a lower long-term mortality (mean follow-up 6.6 years) in patients receiving RA, but no difference in terms of myocardial infarction, stroke, and operative mortality. Numerous observational studies have been conducted to compare RA and RITA. A meta-analysis showed that late death and repeat revascularization rates were lower in patients treated with LITA plus RA (26).
Recently, an analysis performed on 1,493,470 patients from the Society of Thoracic Surgeons database highlighted that an increase in operative mortality was seen in patients receiving BITA but not in those receiving LITA plus RA. Interestingly, the short- and long-term outcomes for BITA were associated with surgeon experience in a U-shaped volume-outcome relation (27). Another report including 59,432 patients found that BITA led to a higher rate of sternal complications but also revealed an equal survival between BITA grafts and LITA plus RA grafts (28).
The main concerns regarding the use of RA graft are related to the aforementioned extent of stenosis of the target coronary vessel, and the spasm tendency of the RA.
Total arterial grafting
Evidence for the existence of the added value of increased arterial revascularization by using 3 arterial conduits is less abundant.
Thus far, only 2 RCTs have compared total arterial revascularization with conventional CABG. In the first trial, 200 patients older than 70 years were randomized to receive either TAG or LITA plus SVG. The TAG group showed a lower rate of graft occlusion, angina recurrence, new percutaneous revascularization. And new myocardial infarction, whereas the mortality at a mean follow-up of 15 months was not different. The use of SVG was found to be a predictor of recurrent angina and graft occlusion (29). The second trial was a pilot feasibility study involving 58 patients, randomized to either TAG or LITA plus SVG. The results showed no difference in in-hospital mortality, stroke, or DSWI between the 2 groups and no difference in graft patency at 6-month follow-up (30).
On the other hand, a meta-analysis by Yanagawa et al., comprising 130,305 patients, showed that TAG was associated with a significantly longer survival compared with SAG or MAG (31). Similarly, a recent meta-analysis by Urso et al., including 18 studies and comparing the TAG versus the non-TAG approach, demonstrated a long-term survival benefit of TAG (32). Interestingly, their meta-regression showed that the benefit seemed to be greater in patients with diabetes mellitus and in those receiving 3 arterial conduits. Moreover, the TAG approach with BITA was associated with a significantly higher survival than TAG without BITA, but had an increased risk of DSWI.
Interestingly, in a study from the Australian and New Zealand Society of Cardiothoracic Surgeons database, SVG was found to be independently associated with poorer survival up to 12.5 years (33).
Multiple arterial grafting versus total arterial grafting
A propensity-score matched study of 11,279 patients showed that main in-hospital outcomes (death, stroke, new onset of atrial fibrillation, kidney dysfunction, blood transfusion, and length of stay) were not different between patients receiving 2 arterial grafts and those receiving 3 arterial grafts (18). Also, no difference was found in the incidence of major adverse cardiac and cerebrovascular events or in long-term survival.
In a recent meta-analysis (34) of 8 propensity score–matched studies, which included 10,287 patients, the use of 3 arterial grafts was associated with a long-term survival benefit, with this advantage being independent of the patient’s gender or diabetes mellitus status. Similar outcomes in terms of major adverse cardiac and cerebrovascular events, death, myocardial infarction, stroke, or repeat revascularization were found in a propensity-matched study using a provincial registry in Ontario between patients receiving 2 or 3 arterial grafts (18).
Multiple and total arterial grafting versus percutaneous coronary intervention
The only RCT comparing percutaneous coronary intervention (PCI) versus CABG performed with more than 1 arterial graft, is the BEST trial (35). Regrettably, the trial was terminated earlier than anticipated due to a slow enrolment. The primary composite endpoint of death, myocardial infarction, and target-vessel revascularization was more frequent in patients undergoing PCI with the use of everolimus-eluting stents than CABG.
A recent network meta-analysis of 25 studies, including a total of 53,239 patients, provided a comparison between MAG and PCI. It found that long-term mortality and need for repeat revascularization were lower in the MAG group while no difference was found in terms of operative mortality and stroke (36). The meta-regression indicated, albeit without significance, that there was a clear trend towards a negative correlation between the number of arterial grafts and the incidence rate ratio for long-term mortality.
Conclusions
The main advantages and disadvantages of multiple and total arterial grafting are shown in Table 1 and Table 2. Abundant evidence is present to support the use of more than 1 arterial graft as a conventional approach in myocardial revascularization. Specifically, observational studies have endorsed this approach although the impact of selection bias on the final results cannot be excluded. There has been a growing interest in the use of propensity-score matched methodology with the aim to control biases related to the observational nature of these studies. Despite this, even the most accurate statistical methodology still cannot address all confounders. As emphasized by Anyanwu and Adams (38), propensity matching is limited when the treatment effect relies strongly on longevity. This statistical methodology does not consider for example “the eye ball test” that is the clinical judgment of physicians deciding to give either a conventional revascularization or a multiple/total arterial revascularization. Another critical point concerns the surgical skills and the experience of the surgeons involved. The authors suggest that heterogeneity in conduit harvesting might be present since SVG harvesting, routinely performed by junior residents or surgeon’s assistants, might involve direct trauma to the vein endothelium and therefore have an impact on graft patency while arterial grafts are usually procured in an atraumatic way by skilled surgeons or surgeon’s assistants. Also, in studies without randomization, the impact of surgeon’s skills is essential, and propensity matching is not able to control it. In a control group for patients usually receiving conventional CABG, grafting is performed by less skilled and/or low-volume surgeons in contrast to patients receiving more demanding arterial grafting, which is performed by highly skilled surgeons who regularly perform that type of surgery.
Table 1
| Categories | Advantages |
|---|---|
| Multiple arterial grafting | Benefit in event-free survival from BITA or SITA plus RA (8)† |
| Survival advantage from BITA versus SITA (12-18)‡ | |
| Reduction of the risk of revascularization (22,23), myocardial infarction (22,23), and cardiac death (22) from the use of RA versus SVG† | |
| Survival advantage from RA versus SVG (25)‡ | |
| Reduction in long-term mortality and repeat revascularization from MAG over PCI (36)‡ | |
| Total arterial grafting | Lower rate of graft occlusion, angina recurrence, new percutaneous revascularization, and new myocardial infarction from TAG versus SITA plus SVG (29)† |
| Survival benefit from TAG over MAG and SAG (31,32,34)‡ | |
| Reduction of the risk of major adverse cardiac and cerebrovascular events, death, myocardial infarction, stroke, and repeat revascularization from TAG versus MAG (18)‡ |
†, evidence from randomized clinical trials; ‡, evidence from observational studies. BITA, bilateral internal thoracic arteries; SITA, single internal thoracic artery; RA, radial artery; SVG, saphenous vein graft; SAG, single arterial grafting; MAG, multiple arterial grafting; TAG, total arterial grafting; PCI, percutaneous coronary revascularization.
Table 2
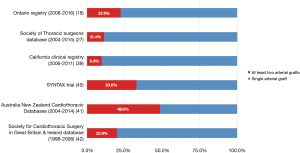
Considering the rate of use of more than 1 arterial graft, a discouraging discrepancy between evidence and clinical practice is present (Figure 1) (18,27,39-42). The reluctance of cardiac surgeons to accept systematic use of more than 1 arterial graft is based on different concerns.
- The main limiting factor for the use of BITA is the perceived increased risk of deep sternal wound infection (DSWI). Traditionally, 2 main methods have been described for the harvesting of internal thoracic arteries: pedicled and skeletonized harvesting. The former involves dissection of the artery together with satellite veins, and lymphatic and adipose tissue, while the dissection of the latter does not include any surrounding tissue and has been described to be a protective factor for DSWI (43). In an ART trial substudy (21), the use of skeletonized BITA did not increase the risk of sternal wound complications compared to pedicled SITA. Also, the skeletonized approach is considered to be able preserve the benefit advantages previously mentioned but will not increase the incidence of DWSI in diabetic patients, as demonstrated in a meta-analysis of 7264 patients (37). Instead, BITA harvesting should be avoided in patients with poorly controlled insulin-dependent diabetes mellitus, chronic obstructive pulmonary disease, and obesity (4).
- Technical difficulties may arise from BITA harvesting due to the fact that, compared to performing anastomosis with SVG, the thoracic artery-coronary anastomosis is more fragile and smaller in size. Other hurdles come when RITA is used as a Y-graft to LITA. BITA grafting requires a higher level of skill and experience, and accurate handling of this technique can be achieved through a steep learning curve. Based on this evidence, the introduction of a coronary surgery-focused sub-specialty is unavoidable.
- The adoption of BITA requires a longer intra-operative time (44), mainly related to the harvesting process rather than the anastomosis itself. Certainly, the use of in-situ internal thoracic artery graft would help shorten the duration of operating time (45).
- The use of RA entails fewer concerns. Firstly, major complications arising from RA harvesting are prevented when modified Allen’s test and ultrasonographic vascular assessment is properly performed to evaluate compensatory ulnar vascularization. Secondly, RA harvesting does not necessarily increase operative time, as it can be performed while harvesting other conduits. The main concern limiting RA adoption is related to competitive flow. As emphasized by current guidelines, RA should be used to target a vessel with at least a stenosis of 70%, and optimally of 90%, of its lumen (4).
The Randomized Comparison of the Clinical Outcome of Single Versus Multiple Arterial Grafts (ROMA) trial results are anticipated to be presented in 2025 and are expected to have substantial value (46). This trial is designed to compare the use of single and multiple arterial grafts in an expected sample size of 4,300 patients.
In conclusion, there is sufficient evidence supporting the use of more than 1 arterial graft when performing coronary artery bypass grafting. In particular, the decision to perform MAG or TAG should depend on a consideration of the patient’s life expectancy, comorbidity, and surgeon’s skills.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shahzad G. Raja) for the series “Coronary Artery Bypass Grafting” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.03.12/coif). The series “Coronary Artery Bypass Grafting” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Green GE, Stertzer SH, Reppert EH. Coronary arterial bypass grafts. Ann Thorac Surg 1968;5:443-50. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986;314:1-6. [Crossref] [PubMed]
- Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts–effects on survival over a 15-year period. N Engl J Med 1996;334:216-9. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. J Am Coll Cardiol 2011;58:e123-210. [Crossref] [PubMed]
- Goldman S, Zadina K, Moritz T, et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149-56. [Crossref] [PubMed]
- Myers WO, Berg R, Ray JF, et al. All-artery multigraft coronary artery bypass grafting with only internal thoracic arteries possible and safe: a randomized trial. Surgery 2000;128:650-9. [Crossref] [PubMed]
- Nasso G, Coppola R, Bonifazi R, et al. Arterial revascularization in primary coronary artery bypass grafting: Direct comparison of 4 strategies--results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg 2009;137:1093-100. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized Trial of Bilateral versus Single Internal-Thoracic-Artery Grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Taggart DP, Lees B, Gray A, et al. Protocol for the Arterial Revascularisation Trial (ART). A randomised trial to compare survival following bilateral versus single internal mammary grafting in coronary revascularisation (ISRCTN46552265). Trials 2006;7:7. [Crossref] [PubMed]
- Gaudino M, Bakaeen FG, Benedetto U, et al. Arterial Grafts for Coronary Bypass: A Critical Review After the Publication of ART and RADIAL. Circulation 2019;140:1273-84. [Crossref] [PubMed]
- Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [Crossref] [PubMed]
- Rizzoli G, Schiavon L, Bellini P. Does the use of bilateral internal mammary artery (IMA) grafts provide incremental benefit relative to the use of a single IMA graft? A meta-analysis approach. Eur J Cardiothorac Surg 2002;22:781-6. [Crossref] [PubMed]
- Weiss AJ, Zhao S, Tian DH, Taggart DP, et al. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:390-400. [PubMed]
- Takagi H, Goto S, Watanabe T, et al. A metanalysis of adjusted hazard ratios from 20 observational studies of bilateral versus single internal thoracic artery coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;148:1282-90. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: a meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Buttar SN, Yan TD, Taggart DP, et al. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419-26. [Crossref] [PubMed]
- Rocha RV, Tam DY, Karkhanis R, et al. Multiple Arterial Grafting Is Associated With Better Outcomes for Coronary Artery Bypass Grafting Patients. Circulation 2018;138:2081-90. [Crossref] [PubMed]
- Gaudino M, Di Franco A, Rahouma M, et al. Unmeasured Confounders in Observational Studies Comparing Bilateral Versus Single Internal Thoracic Artery for Coronary Artery Bypass Grafting: A Meta-Analysis. J Am Heart Assoc 2018;7:e008010. [Crossref] [PubMed]
- Lazar HL. The risk of mediastinitis and deep sternal wound infections with single and bilateral, pedicled and skeletonized internal thoracic arteries. Ann Cardiothorac Surg 2018;7:663-72. [Crossref] [PubMed]
- Benedetto U, Altman DG, Gerry S, et al. Pedicled and skeletonized single and bilateral internal thoracic artery grafts and the incidence of sternal wound complications: Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2016;152:270-6. [Crossref] [PubMed]
- Zhang H, Wang ZW, Wu HB, et al. Radial artery graft vs. saphenous vein graft for coronary artery bypass surgery: which conduit offers better efficacy? Herz 2014;39:458-65. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes SRADIAL Investigators, et al. Radial-artery or saphenous-vein grafts in coronary artery bypass surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Buxton BF, Hayward PA, Matalanis G, et al. 10-year endpoint of RAPCO is reached: clinical and angiographic results of a randomised trial of radial artery versus right internal thoracic artery or saphenous vein for the second graft. Presented at: 96th Annual Meeting of The American Association for Thoracic Surgery; May 14-18, 2016; Baltimore, Md.
- Gaudino M, Rahouma M, Abouarab A, et al. Radial artery versus saphenous vein as the second conduit for coronary artery bypass surgery: a meta-analysis. J Thorac Cardiovasc Surg 2019;157:1819-25.e10. [Crossref] [PubMed]
- Gaudino M, Lorusso R, Rahouma M, et al. Radial Artery Versus Right Internal Thoracic Artery Versus Saphenous Vein as the Second Conduit for Coronary Artery Bypass Surgery: A Network Meta-Analysis of Clinical Outcomes. J Am Heart Assoc 2019;8:e010839. [Crossref] [PubMed]
- Schwann TA, Habib RH, Wallace A, et al. Operative Outcomes of Multiple-Arterial Versus Single-Arterial Coronary Bypass Grafting. Ann Thorac Surg 2018;105:1109-19. [Crossref] [PubMed]
- Goldstone AB, Chiu P, Baiocchi M, et al. Second Arterial Versus Venous Conduits for Multivessel Coronary Artery Bypass Surgery in California. Circulation 2018;137:1698-707. [Crossref] [PubMed]
- Muneretto C, Bisleri G, Negri A, et al. Total arterial myocardial revascularization with composite grafts improves results of coronary surgery in elderly: a prospective randomized comparison with conventional coronary artery bypass surgery. Circulation 2003;108:II29-33. [Crossref] [PubMed]
- Le J, Baskett RJ, Buth KJ, et al. A pilot randomized controlled trial comparing CABG surgery performed with total arterial grafts or without. J Cardiothorac Surg 2015;10:1. [Crossref] [PubMed]
- Yanagawa B, Verma S, Mazine A, et al. Impact of total arterial revascularization on long term survival: a systematic review and meta-analysis of 130,305 patients. Int J Cardiol 2017;233:29-36. [Crossref] [PubMed]
- Urso S, Sadaba R, González JM, et al. Total arterial revascularization strategies: A meta-analysis of propensity score‐matched observational studies. J Card Surg 2019;34:837-45. [Crossref] [PubMed]
- Royse A, Pawanis Z, Canty D, et al. The effect on survival from the use of a saphenous vein graft during coronary bypass surgery: a large cohort study. Eur J Cardiothorac Surg 2018;54:1093-100. [Crossref] [PubMed]
- Gaudino M, Puskas JD, Di Franco A, et al. Three Arterial Grafts Improve Late Survival: A Meta-Analysis of Propensity-Matched Studies. Circulation 2017;135:1036-44. [Crossref] [PubMed]
- Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204-12. [Crossref] [PubMed]
- Gaudino M, Rahouma M, Abouarab A, et al. Meta-Analysis Comparing Outcomes of Drug Eluting Stents Versus Single and Multiarterial Coronary Artery Bypass Grafting. Am J Cardiol 2018;122:2018-25. [Crossref] [PubMed]
- Kajimoto K, Yamamoto T, Amano A. Coronary artery bypass revascularization using bilateral internal thoracic arteries in diabetic patients: a systematic review and meta-analysis. Ann Thorac Surg 2015;99:1097-104. [Crossref] [PubMed]
- Anyanwu AC, Adams DH. Total Arterial Revascularization for Coronary Artery Bypass: A Gold Standard Searching for Evidence and Application. J Am Coll Cardiol 2018;72:1341-5. [Crossref] [PubMed]
- Goldstone AB, Chiu P, Baiocchi M, et al. Second Arterial Versus Venous Conduits for Multivessel Coronary Artery Bypass Surgery in California. Circulation 2018;137:1698-707. [Crossref] [PubMed]
- Head SJ, Parasca CA, Mack MJ, et al. Differences in baseline characteristics, practice patterns and clinical outcomes in contemporary coronary artery bypass grafting in the United States and Europe: insights from the SYNTAX randomized trial and registry. Eur J Cardiothorac Surg 2015;47:685-95. [Crossref] [PubMed]
- Schwann TA, Tatoulis J, Puskas J, et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004-2014: A Tale of 2 Continents. Semin Thorac Cardiovasc Surg 2017;29:273-80. [Crossref] [PubMed]
- Society for Cardiothoracic Surgery in Great Britain and Ireland. 2008 National Adult Cardiac Database Report. Available online: http://www.scts.org.
- Sá MP, Soares EF, Santos CA, et al. Risk factors for mediastinitis after coronary artery bypass grafting surgery. Rev Bras Cir Cardiovasc 2011;26:27-35. [Crossref] [PubMed]
- Tatoulis J, Wynne R, Skillington PD, et al. Total Arterial Revascularization: Achievable and Prognostically Effective-A Multicenter Analysis. Ann Thorac Surg 2015;100:1268-75. [Crossref] [PubMed]
- Raja SG. Total arterial off-pump coronary revascularization: The Holy Grail? Curr Opin Cardiol 2019;34:552-6. [Crossref] [PubMed]
- Gaudino M, Alexander JH, Bakaeen FG, et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial—rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031-40. [Crossref] [PubMed]
Cite this article as: Dimagli A, Benedetto U. Multiple and total arterial coronary artery bypass grafting. AME Med J 2020;5:28.

