
Hybrid coronary revascularization
Introduction
Coronary artery bypass grafting (CABG) continues to remain the gold standard for management of patients with complex multi-vessel coronary artery disease (CAD), with percutaneous coronary intervention (PCI) being an acceptable alternative in patients with a low SYNTAX score (1-4). Additionally, CABG has been shown to be a safe and effective treatment for patients with all severities of left main disease (LMD), while the use of PCI as a therapy for mild to moderate LMD still remains a topic of debate following the conflicting and controversial results of 2 recently concluded large randomized controlled trials (RCTs) (5-7). One of the most important advantages of the CABG procedure is the use of the left internal thoracic artery graft (LITA) to left anterior descending artery (LAD), which provides incomparable long-term clinical benefits that seem to increase during the second decade after surgery (8). It has not only been found to be an independent predictor of long-term survival (9), but absence of its use is associated with an increased risk of reoperation as well (10).
The development and widespread use of the minimally invasive direct coronary artery bypass (MIDCAB), which involves a single LITA bypass graft to the LAD through a left small anterior thoracotomy approach for isolated LAD disease, has been associated with excellent long-term results (11). Furthermore, newer generation drug-eluting stents (DES) have been associated with lower rates of restenosis and thrombosis (12) due to tremendous advancements in delivery systems (radial approach), imaging (intravascular ultrasound) and physiological assessments (fractional/instantaneous flow reserve). Nevertheless, the rate of target vessel revascularization (TVR) for the LAD is still significantly greater following PCI with DES as compared to a MIDCAB procedure (13). These developments in surgical and interventional revascularization have given impetus to another revascularization strategy called hybrid coronary revascularization (HCR), which was first reported by Angelini and Calafiore as early as 1996 (14). Historically, it is a term applied to planned multi-vessel revascularization achieved through a single- or multi-staged revascularization strategy involving a MIDCAB operation and PCI of the non-LAD vessels. It serves as an additional weapon in the armamentarium of cardiologists and cardiac surgeons that not only aids in reducing postoperative morbidity without jeopardizing the potential long-term benefits of CABG surgery, but can also promote the use of revascularization in selected patients previously rejected for PCI of the LAD artery due to disease complexity or for surgery based on the presence of multiple comorbidities. Additionally, it provides the benefits of a minimally invasive surgical approach such as reduced postoperative pain and bleeding, early recovery and cosmesis. It, however, requires a very closed-knit Heart Team that can determine the indications/patient selection, the appropriate sequence and timing of the type of revascularization, and anticoagulation management, all of which are addressed in the current review.
Patient selection
HCR is not yet used as the standard of care for patients with CAD and is currently reserved only for a select group of patients. The most recent guidelines for myocardial revascularization provide a Class IIb level B recommendation for hybrid procedures due to a lack of large multicentre randomized studies demonstrating its equivalence to the conventional techniques of revascularization (15). The chief criterion for decision-making in HCR is the coronary anatomy of the patient. It is ideally suited for patients with multivessel CAD with complex lesions or occlusion of the LAD that is not deemed suitable for PCI and relatively mild to moderate disease involving the non-LAD vessels that are easily amenable to PCI. However, such patients could also be well-served with a conventional CABG operation. Therefore, HCR seems to be a good option in patients with such coronary anatomy, if they have multiple comorbidities making them high-risk candidates for conventional CABG procedures or have a high likelihood of sternal wound complications or are wheel-chair/walker-dependent (Table 1). HCR can also be offered as an acceptable alternative to conventional surgery to patients who prefer or have an outright desire for the least invasive approach possible.
Table 1
| Rationale for selection of HCR in surgical candidates |
| High predicted operative mortality following conventional CABG determined by calculation of the EuroSCORE II or the STS score |
| Anatomical factors |
| Complex LAD lesions with simple focal lesions in the right coronary and/or circumflex arteries |
| Poor target vessels for CABG and lack of conduits |
| Severely calcified/porcelain aorta |
| Significant risk factors for sternal wound infections and mediastinitis |
| Insulin-dependent diabetes mellitus |
| Obesity |
| Patients dependent on walkers, crutches or wheel-chairs |
| Skeletal abnormalities such as severe osteoporosis or osteogenesis imperfecta |
| Elderly patients with severe frailty |
| Rationale for selection of HCR in patients suited for PCI |
| To provide the long-term survival benefit of LITA-LAD graft to patients with multivessel disease and low SYNTAX scores, who are suitable for a MIDCAB procedure |
CABG, coronary artery bypass grafting; HCR, hybrid coronary revascularization; LAD, left anterior descending; PCI, percutaneous coronary intervention; LITA, left internal thoracic artery; MIDCAB, minimally invasive direct coronary artery bypass.
A completely different perspective that was first conceived by Puskas and colleagues would be to consider it as an option in patients who lie in the grey zone with respect to the SYNTAX score and may actually be anticipated to have greater survival benefit with CABG than with PCI, but who often undergo PCI instead of CABG, chiefly due to the invasiveness of the latter (16,17). The excellent long-term results of the LITA graft to the LAD are irrefutable. HCR will, therefore, provide complete revascularization through PCI of non-LAD vessels, which is the least invasive form of revascularization therapy combined with the long-term survival benefit of the LITA-LAD graft through a left anterior small thoracotomy with or without robot-assist or a totally endoscopic robot-assisted approach, which is currently the least invasive surgical revascularization technique available.
Although HCR appears to be a very attractive strategy for revascularization of patients with multivessel CAD, there are certain absolute contraindications that need to be reckoned (Table 2). MIDCAB should be avoided in emergent situations as LITA harvest usually takes longer than that through a sternotomy and in the event of a sudden hemodynamic collapse, a sternotomy allows for an effective cardiac massage and expeditious establishment of cardiopulmonary bypass (CPB), which may be a challenging prospect in patients undergoing MIDCAB. Patients with severe respiratory disorders do not tolerate single-lung ventilation and are, therefore, not good candidates for a MIDCAB procedure. Additionally, presence of a deep intramyocardial LAD, severe left subclavian artery stenosis, left-sided arteriovenous fistulas for hemodialysis and/or morbid obesity and patients who have undergone previous left-sided thoracic surgeries are relative contraindications for a MIDCAB and, thereby, a HCR procedure. Similarly, some patients may have one or more non-LAD vessels unsuitable for PCI (Table 2). Such patients would also be a contraindication for HCR procedures.
Table 2
| Contraindications for a MIDCAB procedure |
| Absolute contraindications |
| Non-graftable LAD |
| Severe chest deformities |
| Severe pleural adhesions which can be diagnosed on a high-resolution computed tomogram in suspected cases with previous: |
| Left-sided thoracic surgery |
| High-dose chest radiation |
| Pleural tapping or chest drains |
| History of pleural diseases |
| Intolerance to or inability to establish single-lung ventilation |
| Severe chronic obstructive pulmonary disease |
| FEV1 <50% |
| Narrow trachea precluding use of a double-lumen endotracheal tube |
| Severe left subclavian artery stenosis/occlusion |
| Relative contraindications |
| Deep intramyocardial or severely calcified LAD |
| Body mass index >40 kg/m2 |
| Contraindications for PCI of non-LAD vessels |
| Absolute contraindications |
| Severe peripheral vascular disease precluding vascular access (including radial access) |
| Vessel size: <2.0 mm or severely ectatic vessel |
| Tortuous vessels that could preclude safe stent placement |
| Relative contraindications |
| Anatomical factors |
| Chronically occluded vessels |
| Multi-segment and/or diffuse disease |
| Severely calcified lesions, long lesions, and bifurcated lesions |
| Serum creatinine ≥200 μmol/L |
FEV, forced expiratory volume; HCR, hybrid coronary revascularization; LAD, left anterior descending; MIDCAB, minimally invasive direct coronary artery bypass; PCI, percutaneous coronary intervention.
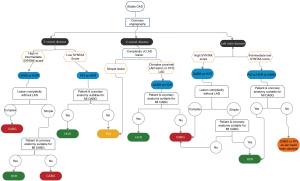
Figure 1 depicts an algorithm for selection of patients with stable CAD for HCR and is chiefly based on coronary anatomy of patients. The aforementioned indications and contraindications should be taken into consideration during the decision-making process. We believe that the final decision to perform a HCR procedure should be a Heart Team recommendation that is individualized not only to the patient, but is also dependent on the operating surgeon, the cardiologist, the anesthesiologist and the intensivists based on their experience and ability to manage complex coronary anatomy and significant patient comorbidities (18).
Sequence of revascularization
The sequence of revascularization remains controversial till date due to the lack of strong evidence supporting one viewpoint or the other. One of the 3 options is essentially possible, namely, minimally invasive surgical revascularization followed by PCI of non-LAD vessels, PCI of non-LAD vessels followed by minimally invasive surgical revascularization and a simultaneous procedure i.e. both surgery and PCI performed in one stage. Most of HCR procedures performed in different studies were performed in stages, rather than simultaneously (14,17,19,20), indicating a preference of different Heart Teams for this approach. Each option has its benefits and drawbacks that have been addressed below.
Surgical revascularization followed by PCI of non-LAD vessels
The vast majority of HCR procedures are performed as a staged approach, in which the LITA to LAD grafting is performed in the first stage with a minimally invasive approach followed by PCI of the non-LAD vessels at the second stage (21,22). No consensus exists on the timing of the second stage either. Most of the PCI procedures are performed during the same hospital stay (23,24), but it finally depends on the Heart Team decision, which in turn depends on the severity of residual disease in the untreated vessels. On the contrary, some cardiologists advocate PCI at a later date, when the postoperative hypercoagulable state following surgery has abated, especially when it is performed off-pump (25). The major advantage of this approach is that the most important coronary artery, the LAD, is revascularized first, which obviously provides protection to more than 50% of the myocardium during PCI of the non-LAD target vessels. Additionally, the quality and function of the LITA-LAD graft can be assessed during the PCI procedure. Furthermore, dual antiplatelet therapy can be started once the risk of perioperative bleeding is reduced, thereby, optimizing conditions for a PCI procedure. This could potentially reduce the possibility of stent thrombosis following PCI. The only drawback is incomplete revascularization during surgery, which could result in ischemia driven hemodynamic compromise or arrhythmias particularly if the non-LAD vessels have severe disease (26).
PCI of non-LAD vessels followed by surgical revascularization
The PCI-first approach is uncommon and is frequently used in daily practice in urgent and emergent situations, when PCI is performed for non-LAD culprit lesions following an acute coronary syndrome and a minimally invasive LITA-LAD graft is performed at a later date. We, however, believe that this does not come under the realm of planned HCR, but rather a compelling situation that forces the physicians into a HCR procedure. The lower affinity of physicians for this approach in stable CAD patients is manifold. First, the LAD, which supplies the largest myocardial area, remains untreated, thus posing an increased risk for a life-threatening myocardial infarction (MI), especially in tight proximal LAD lesions. Second, the patients receive dual antiplatelet medication following PCI, which increases the risk of postoperative bleeding and re-exploration (27). Third, discontinuation of DAPT, use of procoagulant factors/products and a hypercoagulable state in the perioperative period increases the risk of stent thrombosis (28). Fourth, the LITA-LAD graft cannot be assessed. The only benefit is that in the event of failed or complicated PCI of one or more of the non-LAD vessels, which is relatively uncommon due to the lesser complexity of these lesions, the option of performing complete revascularization with conventional CABG with or without the management of the complications is still available.
Simultaneous PCI and surgical revascularization
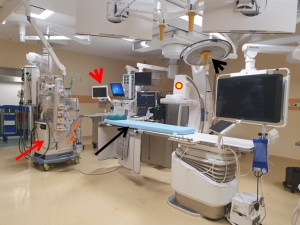
Simultaneous revascularization procedures typically involve performance of a LITA-LAD bypass graft through a minimally invasive approach followed by immediate revascularization of the non-LAD vessels with PCI. The prerequisite for such a strategy is a hybrid operating room (Figure 2), which is designed to enable clinicians to perform simple and complex interventional procedures and surgeons to execute either conventional or minimally invasive techniques. It obviously has all the aforementioned advantages of the staged surgery-first strategy with some additional benefits such as immediate assessment of the LITA-LAD graft, which makes graft revision in case of inadequacies still feasible during the same procedure. Similarly, PCI failures or complications can also be addressed instantaneously. Additionally, a single stage procedure reduces postoperative morbidity (29) and facilitates earlier discharge from the hospital and thereby improves patient satisfaction and recovery. Nevertheless, a one-stage approach is also fraught with certain drawbacks in addition to those already cited for the surgery-first scenario. It involves longer operative and anesthesia times and higher costs due to the use of a hybrid suite. Although the potential for acute kidney injury could be greater in patients undergoing CABG with CPB and simultaneous PCI due to contrast-induced renal insult, it is less likely to occur in the modern era when LITA-LAD is performed off-pump through a minimally invasive approach and less nephrotoxic contrast solutions are available (29). Furthermore, special logistical issues such as coordination of the surgical and interventional cardiology teams and availability of the hybrid operating room could also pose challenges to the organization of a single-staged approach (30,31). The Heart Team would have to play an active role in the execution of this strategy.
Surgical options in HCR
The main advantage of the hybrid approach is avoiding a median sternotomy and the use of CPB for surgical revascularization. Therefore, we believe that HCR procedures should not encompass off-pump LITA-LAD graft through a median sternotomy and PCI of the non-LAD vessels, even though by definition it fits into the realm of a hybrid procedure. We are of the opinion that once a median sternotomy has been performed, complete surgical revascularization should be achieved, which realistically should not be difficult considering the lack of complexity of non-LAD lesions. As a result, only minimally invasive surgical options in the context of a hybrid approach have been addressed below.
MIDCAB
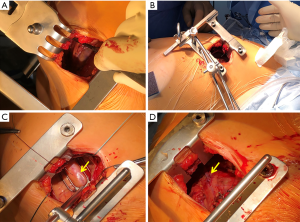
A MIDCAB procedure entails performance of a LITA-LAD graft through a left anterior small thoracotomy. Specialized retractors that facilitate the harvest of the LITA under direct vision are commercially available (Figure 3A). Following LITA harvest, the LITA-LAD anastomosis is performed off-pump, most commonly with the help of mechanical pressure stabilizers that are designed for repeated use (Figure 3B,C,D). Nonetheless, commercially available non-reusable suction stabilizers could also be used in patients with a difficult anatomy. MIDCAB is the preferred operation at our institution. Over the last 2 decades, more than 2500 procedures have been performed with an in-hospital mortality of just under 1% (all comers). We have previously demonstrated that long-term survival is good and re-intervention rates are low (11). It is commonly used as the surgical procedure of choice for HCR (29,32), because it can be executed relatively quickly, is reproducible and has a smaller learning curve as compared to other techniques. It has a smaller wound that facilitates improved healing and early recovery (33). Its efficacy and safety have been shown to be comparable to both CABG and PCI (34-36). Moreover, procedural success is almost 100% and chronically occluded LADs do not have a negative impact on long-term survival (37). The only drawback is that postoperative pain and the requirement for pain medications is higher following a LITA-LAD graft through a small anterior thoracotomy than a mini-sternotomy (38).
Endoscopic atraumatic coronary artery bypass (EACAB)
A modification of the MIDCAB procedure is EACAB, in which the LITA is harvested with the help of an endoscope and a harmonic scalpel (39). The main advantages of this technique over MIDCAB are lesser chest wall retraction and costal cartilage dislocation during LITA harvest resulting in lesser postoperative pain. Additionally, it enables complete dissection of the LITA, especially in its inferior segment, which could be challenging while using direct vision for its harvest. EACAB has been associated with excellent perioperative outcomes with a conversion rate to sternotomy of 1% and LITA injury in 0.5% of patients. The long-term outcomes are equally promising with an overall death and target vessel revascularization rate of 2.7% each (39). Use of a 3D endoscope may be able to enhance the vision as it can obtain stereoscopic vision with higher resolution and may lead to shorter operating times (40).
Robot-assisted coronary artery bypass (RACAB)
RACAB is another modification of the MIDCAB procedure that involves LITA harvest with a robot. The pericardiotomy, identification of the LAD and the exact site of anastomosis are also robotically accomplished. Thereafter, a hand-made anastomosis is performed through a small non-rib-spreading 3- to 4-cm anterolateral thoracotomy without the use of CPB (41). The main advantage of robotic assistance is that it provides a high-definition intrathoracic exposure and 3-D telemanipulation, which facilitates the complete takedown of the LITA without distorting the thoracic cage. Additionally, its further aids in identifying the exact spot on the LAD that is best suitable for the anastomosis, thereby, guiding the surgeon to determine the precise site of entry into the thorax so that the anastomosis can be performed with minimal rib-spreading. This further leads to less tissue trauma, lower transfusion rate, and reduction in pain, which translates into immediate post-procedure extubation, shorter hospital stay, and early return to normal physical activities (42,43). It is associated with a low perioperative mortality, reduced re-exploration and acceptable early patency rates (44).
Totally endoscopic coronary artery bypass (TECAB)
TECAB is the least invasive CABG procedure that entails performance of a LITA-LAD grafting without any surgical incision. It involves a totally endoscopic harvest of the LITA followed by a LITA-LAD anastomosis that can be hand-sewn endoscopically or be facilitated by a distal anastomotic device and can be performed with or without CPB. It is arguably the commonest cardiac surgical procedure performed robotically. It can be considered the final frontier of CABG surgery. TECAB, which was first reported by Loulmet in 1999 (45), was initially performed with the automated endoscopic system for optical positioning (AESOP) and the Zeus Robotic Surgical System that gradually evolved into the da Vinci Surgical System and its various models such as the daVinci S, daVinci Si and daVinci Xi systems (Intuitive Surgical, Inc., Sunnyvale, CA, USA) that are available today (46). It basically consists of a surgical console, where the surgeon is seated, a computer controlled system, which digitizes the surgeon’s hand movements and a slave unit that consists of robotic manipulators with three or four arms that are fixed to the operating table. Additionally, it has a video tower with screens that provides the surgeon’s view to the rest of the surgical team. The main disadvantages are the steep learning curve, the one-time cost of the main unit and the recurring costs of disposables, and the longer procedural times as compared to other forms of minimally invasive surgeries, which have restricted the widespread use of TECAB. Nevertheless, excellent vision and magnification, elimination of tremors, presence of tactile sensation in the most modern versions of the robot and the lack of a surgical incision and chest distortion during surgery are some of the benefits associated with TECAB.
Anticoagulation management
Management of anticoagulation in HCR procedures is akin to performing a fine balancing act between maintenance of adequate levels of antiplatelet action to prevent stent thrombosis after PCI while preserving just enough levels of coagulation function to reduce the risk of perioperative bleeding.
Maintaining this balance is the easiest when minimally invasive LITA-LAD grafting is performed prior to PCI, which is the commonest strategy utilized (47,48). CABG is performed under aspirin therapy and a second antiplatelet medication is added postoperatively once the risk of bleeding appears to have subsided. In the PCI-first, but staged strategy, the patients are already on a dual antiplatelet therapy (DAPT) prior to surgery, which makes the balancing act that much more difficult. Discontinuation of ticagrelor 72 hours before surgery, as opposed to 5 days, does not increase the incidence of major bleeding complications after CABG as opposed to clopidogrel (49). However, discontinuation <72 hours prior to surgery is associated with an increased incidence of bleeding complications for both, ticagrelor and clopidogrel-treated patients (49). However, discontinuation of P2Y12 inhibitors followed by replacement therapy with low molecular weight or intravenous heparin has not been investigated for HCR procedures. Therefore, CABG is often being performed without interruption of DAPT (50). In the one-stage HCR procedures, numerous variations can be used in implementation of DAPT. The best option would be to perform minimally invasive LITA-LAD grafting under aspirin therapy alone, followed by administration of a loading dose of P2Y12 inhibitors after completion of surgery but prior to PCI (51) or immediately after PCI (30).
Results
Whether HCR becomes mainstream line of treatment for patients with CAD depends on the strength of the evidence that supports its use. Ever since the first report of HCR in 1996 (14), several retrospective studies have been published in literature, more so in the last decade probably due to tremendous advancements that have been made in the field of minimally invasive CABG and PCI. However, there is marked lack of homogeneity amongst the numerous studies that have reported on the outcomes of HCR, partly due to the differences in the timing and sequence of the type of revascularization (PCI or CABG) and partly due to the variety in the procedural techniques used to perform CABG or PCI. The most robust evidence required to establish best practice guidelines is provided by RCTs followed by meta-analyses that compare the newer techniques to the well-established and proven conventional methods of treatment. We have, therefore, mainly included RCTs, meta-analyses and major observational studies in the current review.
Till date, very few small RCTs relating to HCR exist in literature. The POL-MIDES (HYBRID) (Safety and Efficacy Study of Hybrid Revascularization in Multivessel Coronary Artery Disease) was the first RCT that compared outcomes between HCR and CABG in 200 patients with multivessel CAD involving the LAD and a critical (>70%) lesion in at least 1 major epicardial vessel (except the LAD), who were randomly assigned to one of the 2 revascularization strategies (52). Only 6% of the patients in the HCR group crossed over to the CABG group. The study reported no differences in mortality (2.9% vs. 2.0%, P=NS), MI (3.9% vs. 6.1%, P=NS), major bleeding (2.0% vs. 2.0%, P=NS) and repeat revascularization (0.0% vs. 2.0%, P=NS) rates between HCR and CABG at the end of 1 year. It not only demonstrated the feasibility of HCR in patients with multivessel CAD, but also showed no significant rise in adverse events with a MIDCAB-first strategy. Furthermore, the 5-year outcomes of this trial validated at least the mid-term efficacy of HCR (53). All-cause mortality (6.4% vs. 9.2%; P=0.69), MI (4.3% vs. 7.2%; P=0.30), repeat revascularization (37.2% vs. 45.4%; P=0.38), stroke (2.1% vs. 4.1%; P=0.35), and major adverse cardiac and cerebrovascular events (MACCE) (45.2% vs. 53.4%; P=0.39) at 5-year follow-up were similar in the HCR and CABG groups.
The first prospective RCT to compare the safety and efficacy of all the modes of revascularization was the HVRES study (Hybrid coronary REvascularization Versus Stenting or Surgery), in which 155 patients were randomly allocated on a 1:1:1 ratio to CABG or HCR or PCI with everolimus-eluting CoCr stents (54). Residual ischemia, as calculated by single-photon emission computed tomography, at 12 months was similar between groups and met non-inferiority criteria. The study also revealed no differences in freedom from MACCE or its individual components at 12 months, but total TVR rate numerically favored CABG (CABG: 4.0%; HCR: 13.5%; PCI: 7.0%; P=0.095). Nonetheless, hospital stay and sick leave were the lowest in patients undergoing PCI followed by HCR and CABG. However, it is noteworthy that the patients included in the above trials were amenable to both, PCI and CABG and the mean SYNTAX score was low to intermediate ranging between 19.3±3.0 and 23.4±6.3. Only 8 patients in the POL-MIDES had a SYNTAX score >33. Therefore, HCR has not yet been shown to be equivalent to CABG in patients with severe multivessel CAD. Such patients are still served best with CABG, unless they are deemed inoperable or extreme high risk for surgery, in which case they may be treated with other modalities of revascularization with the inherent risk of incomplete revascularization. Another small single center pilot randomized trial—the MERGING study (The Myocardial hybrid revascularization versus coronary artery bypass GraftING for complex triple-vessel disease), which randomized 60 patients in a 2:1 ratio to HCR and conventional CABG, recently reported a higher rate of unplanned repeat revascularization in the HCR group (14.5% vs. 5.9%) 2 years after the procedures (55). The study, however, revealed that unplanned revascularization was higher (9.5%) for target lesions previously treated with CABG than PCI (2.6%) within the HCR group. Additionally, the only deaths in the entire study were observed in 2 patients in the HCR group following LITA-LAD grafting but prior to PCI—one due to ventricular fibrillation 4 hours following surgery and the other due to LITA occlusion. This study underlines the importance of LITA-LAD grafting and the technical competency and perfectionist attitude of the surgeons performing the MIDCAB component of the HCR procedure.
Due to the small number of patients enrolled in the above-mentioned RCTs, inferences derived from these studies always remain debatable. Meta-analyses could potentially validate or oppose conclusions drawn from such RCTs. In a meta-analysis including 1,190 patients from 6 observational studies, Harskamp and colleagues validated the major findings of the afore-mentioned RCTs (56). On comparing 366 patients undergoing HCR with 824 undergoing off- or on-pump CABG, they found that HCR patients required lesser post-procedural blood transfusions, shorter hospital stay, and could return to work earlier than those undergoing conventional CABG. However, no significant differences were found in MACCE rates during hospitalization [odds ratio (OR): 0.63, 95% confidence interval (CI): 0.25–1.58, P=0.33] and at 1-year follow-up (OR: 0.49, 95% CI: 0.20–1.24, P=0.13). Repeat revascularization rates were higher among patients treated with HCR. A more recent analysis by Sardar et al. which included 2,245 patients undergoing HCR or CABG from one RCT and 7 observational studies, further confirmed the above findings (57). The risk of the composite of death, stroke and MI was similar between HCR and CABG (3.6% and 5.4%, respectively; OR: 0.53; 95% CI: 0.24–1.16). In an even bigger pooled analysis involving 14 studies and 4260 patients undergoing HCR (n=1350) and CABG (n=2910), Reynolds and coworkers analyzed and compared hospital costs in addition to the common clinical outcomes between CABG and HCR. While the latter were similar to the previous studies, the mean difference in hospital costs was almost 4,000 US dollars, being significantly higher in patients undergoing HCR (95% CI: 2.57–5.37, P<0.0001) (58). The added costs of radiographic instruments and stent implantation could be responsible for greater expenses associated with HCR (59), which could, nonetheless, be partially offset by the improved resource utilization such as reduction in blood transfusion, ventilator time, shorter hospital stay observed following this strategy (47,60).
One of the landmark papers involving HCR was that describing a prospective multicenter observational US cohort study funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (17). It is one of the very few studies that compared outcomes in patients with multivessel CAD undergoing HCR to those undergoing multi-vessel PCI with DES. Of note again was the mean SYNTAX score, which was low at 19.7±9.6. The risk-adjusted MACCE rates were similar between HCR and PCI groups 12 months following intervention [hazard ratio (HR): 1.063; P=0.8]. An intriguing fact was that at 18 months of follow-up, event-free survival in the PCI-only arm slid downwards in comparison to the HCR arm causing the MACCE-free survival curves to diverge in favor of the latter. This difference in outcomes, however, did not reach statistical significance (HR: 0.9; P=0.5). This study was used to stipulate the groundwork and establish the eligibility criteria for the Hybrid Coronary Revascularization Trial, which was a multicenter, randomized comparative effectiveness trial of HCR and multivessel PCI funded by the National Heart, Lung, and Blood Institute (NCT03089398) (61). Unfortunately, the trial had to be stopped following withdrawal of funding due to a slow enrollment rate. Most retrospective studies have reported comparisons between HCR and CABG with a single internal thoracic artery (ITA) and veins (50,56). One of the main justifications for HCR is the higher attrition rate of venous grafts within the first year following CABG when compared to the newer generation DES. Use of multiple arterial grafts, especially bilateral ITAs, has been shown to be associated with improved mid- to long-term outcomes following CABG (62-64). Besides, long-term and intervention-free survival (up to at least 8 to 9 years follow-up) following multiple arterial bypass grafting is superior to PCI with DES (65,66). Therefore, caution should be exercised when selecting patients for HCR, particularly those who have the potential of significantly benefitting from multiple arterial grafting. Nevertheless, if appropriately selected (older age, lower BMI, previous PCI, hypertension, diabetes and stable 2-vessel disease), Rosenblum and colleagues demonstrated that HCR can achieve a comparable mid-term survival to conventional CABG performed with bilateral ITAs (HR: 1.05; 95% CI: 0.48–2.29; P=0.91) (19). Only long-term follow-up studies in the future will be able to provide evidence to whether HCR can stand the test of time and remain comparable to multiple arterial grafting with bilateral ITAs even in the second decade of life following revascularization.
Finally, it would only be prudent to assess the impact of improvements in techniques of conventional and minimally invasive CABG and technology in stent manufacturing on HCR. Since majority of minimally invasive LITA-LAD procedures are performed without the use of CPB, a comparison of HCR with off-pump CABG is warranted. The Emory university group compared off-pump CABG to HCR performed chiefly with EACAB or RACAB and DES in patients with multivessel and LMD (50,67). In patients with multivessel disease (HCR n=147, off-pump CABG n=588), the matched groups did not show any differences in in-hospital MACCE (2% in both groups) and 5-year survival (off-pump CABG 84.3% vs. HCR 86.8%; P=0.61). However, repeat revascularization at median follow up of 3.2 years was higher for HCR than for off-pump CABG (12.2% vs. 3.7%; P<0.001) (50). The smaller LMD study (HCR n=27, off-pump CABG n=81) similarly demonstrated no difference in the postoperative complication rate between HCR and off-pump CABG. HCR was associated with a higher but non-significant number of repeat revascularization procedures. Larger observational studies or RCTs are necessary to determine the impact of HCR in LMD. A more recent study comparing HCR with RACAB to off-pump CABG for double vessel disease for the first time showed that though HCR was associated with higher in-hospital repeat revascularization rates than off-pump CABG (3.4% vs. 0%) probably due to protocol-driven angiography of the LITA-LAD graft performed during the staged-PCI procedure, those at a median follow-up of ~7 years were similar (91% vs. 92%; P=0.80) (20). Furthermore, freedom from angina was better in the HCR group (90% vs. 73%; P<0.0001) and long-term survival also showed a trend in favor of HCR (96% vs. 85%; P=0.054). These findings are surprising for several reasons. Native CAD progression has been found to be accelerated following PCI (68), especially in patients with multivessel disease (69). It leads to recurrent angina (70) and is associated with an increased risk of MI and death (71). In addition, problems occurring in grafts to non-LAD vessels are very often benign and clinically silent as long as acceptable native coronary flow is maintained. The POL-MIDES study reported no spontaneous MIs or repeat revascularizations, despite 21% rate of non-LAD graft occlusions noted on protocol-driven angiography in the CABG group at 1-year follow-up (72). Contrarily, acute stent occlusion due to thrombosis often results in MI. Therefore, the results of the afore-mentioned study should be perceived with caution and may be applied chiefly to patients with simple double vessel disease and not be used as a license to perform HCR for patients with three-vessel and/or severe CAD.
Future perspectives
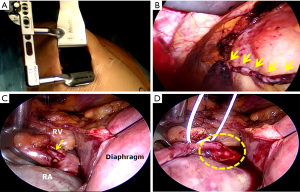
One of the most important goals of the proponents of HCR in the near future should be to establish reliable evidence that would clearly define the patients who would benefit the most from this procedure and the best sequence and timing for surgical and interventional revascularization. A concerted effort on the part of cardiologists and cardiac surgeons would be required to successfully conduct and execute a large multicenter RCT. The indications for HCR can be further expanded to patients with complex left-sided and simple right-sided CAD by performing minimally invasive multivessel off-pump multiple arterial grafting of the left coronary system (73-75) followed by PCI of the right coronary artery (RCA) lesion. This would provide younger patients with the survival benefit of bilateral ITAs and a minimally invasive sternotomy-sparing approach without the risk of sternal wound infection (73). Another configuration of HCR that could be utilized in a very select group of patients is minimally invasive right ITA-RCA grafting and PCI of simple left-sided lesions. Balkhy et al. have recently reported on a series of 16 patients, who underwent robotic beating-heart TECAB with the right ITA to the RCA without any mortality or major postoperative complications (76). Our group has performed 15 so-called right-sided MIDCAB procedures performed through a small right anterior thoracotomy (without thoracosope or robot-assist) most often for occluded proximal RCA lesions that are not amenable to or have failed PCI (Figure 4).
Conclusions
HCR is a promising revascularization strategy that has been shown to be safe, feasible, and effective in a select group of patients, particularly with low to intermediate SYNTAX scores. It is associated with significant early post-procedural benefits such as reduced bleeding, shorter hospital stay and quicker recovery as compared to conventional CABG. It, however, does increase in-hospital costs that could potentially be compensated for if performed more routinely. Although some evidence exists with regard to equivalent mid-term outcomes to conventional CABG, there remains a dire need for well-designed RCTs to evaluate the effectiveness of HCR and thereby define its role in future revascularization strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shahzad G. Raja) for the series “Coronary Artery Bypass Grafting” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-88/coif). The series “Coronary Artery Bypass Grafting” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629-38. [Crossref] [PubMed]
- Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: Final five-year followup of the SYNTAX trial. Eur Heart J 2014;35:2821-30. [Crossref] [PubMed]
- Park SJ, Ahn JM, Kim YHBEST Trial Investigators, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204-12. [Crossref] [PubMed]
- Thuijs DJFM, Kappetein AP, Serruys PWSYNTAX Extended Survival Investigators, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325-34. [Crossref] [PubMed]
- Mäkikallio T, Holm NR, Lindsay MNOBLE Study Investigators, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet 2016;388:2743-52. [Crossref] [PubMed]
- Holm NR, Mäkikallio T, Lindsay MM, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020;395:191-9. [Crossref] [PubMed]
- Stone GW, Kappetein AP, Sabik JFEXCEL Trial Investigators, et al. Five-Year Outcomes after PCI or CABG for Left Main Coronary Disease. N Engl J Med 2019;381:1820-30. [Crossref] [PubMed]
- Boylan MJ, Lytle BW, Loop FD, et al. Surgical treatment of isolated left anterior descending coronary stenosis. Comparison of left internal mammary artery and venous autograft at 18 to 20 years of follow-up. J Thorac Cardiovasc Surg 1994;107:657-62. [Crossref] [PubMed]
- Cosgrove DM, Loop FD, Loop FD, et al. Predictors of reoperation after myocardial revascularization. J Thorac Cardiovasc Surg 1986;92:811-21. [Crossref] [PubMed]
- Cameron A, Davis KB, Green GE, et al. Clinical implications of internal mammary artery bypass grafts: the Coronary Artery Surgery Study experience. Circulation 1988;77:815-9. [Crossref] [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single-center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [Crossref] [PubMed]
- Alexander JH, Hafley G, Harrington RA, et al. PREVENT IV Investigators. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA 2005;294:2446-54. [Crossref] [PubMed]
- Raja SG, Uzzaman M, Garg S, et al. Comparison of minimally invasive direct coronary artery bypass and drug-eluting stents for management of isolated left anterior descending artery disease: a systematic review and meta-analysis of 7,710 patients. Ann Cardiothorac Surg 2018;7:567-76. [Crossref] [PubMed]
- Angelini GD, Wilde P, Salemo TA, et al. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 1996;347:757-8. [Crossref] [PubMed]
- Sousa-Uva M, Neumann FJ, Ahlsson A, et al. ESC Scientific Document Group 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4-90. [Crossref] [PubMed]
- Puskas JD, Pawale A, Sharma SK. Hybrid coronary revascularization: a new treatment paradigm for selected patients with multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1284-6. [Crossref] [PubMed]
- Puskas JD, Halkos ME, DeRose JJ, et al. Hybrid Coronary Revascularization for the Treatment of Multivessel Coronary Artery Disease: A Multicenter Observational Study. J Am Coll Cardiol 2016;68:356-65. [Crossref] [PubMed]
- Head SJ, Kaul S, Mack MJ, et al. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur Heart J 2013;34:2510-8. [Crossref] [PubMed]
- Rosenblum JM, Harskamp RE, Vassiliades TA, et al. Hybrid coronary revascularization versus coronary artery bypass surgery with bilateral or single internal mammary artery grafts. J Thorac Cardiovasc Surg. 2016;151:1081-9. [Crossref] [PubMed]
- Hage A, Giambruno V, Jones P, et al. Hybrid Coronary Revascularization Versus Off-Pump Coronary Artery Bypass Grafting: Comparative Effectiveness Analysis With Long-Term Follow-up. J Am Heart Assoc 2019;8:e014204. [Crossref] [PubMed]
- Harskamp RE, Zheng Z, Alexander JH, et al. Status quo of hybrid coronary revascularization for multi-vessel coronary artery disease. Ann Thorac Surg 2013;96:2268-77. [Crossref] [PubMed]
- Ejiofor JI, Leacche M, Byrne JG. Robotic CABG and hybrid approaches: The current landscape. Prog Cardiovasc Dis 2015;58:356-64. [Crossref] [PubMed]
- Voudris K, Avgerinos DV, Feldman D, et al. Hybrid coronary revascularization: present indications and future perspective. Curr Treat Options Cardiovasc Med 2015;17:364. [Crossref] [PubMed]
- Verhaegh AJ, Accord RE, van Garsse L, et al. Hybrid coronary revascularization as a safe, feasible, and viable alternative to conventional coronary artery bypass grafting: what is the current evidence? Minim Invasive Surg 2013;2013:142616. [Crossref] [PubMed]
- Kon ZN, Brown EN, Grant MC, et al. Warm ischemia provokes inflammation and regional hypercoagulability within the heart during off-pump coronary artery bypass: a possible target for serine protease inhibition. Eur J Cardiothorac Surg 2008;33:215-21. [Crossref] [PubMed]
- Leyvi G, Dabas A, Leff JD. Hybrid Coronary Revascularization - Current State of the Art. J Cardiothorac Vasc Anesth 2019;33:3437-45. [Crossref] [PubMed]
- Kim JH, Newby LK, Clare RM, et al. Clopidogrel use and bleeding after coronary artery bypass graft surgery. Am Heart J 2008;156:886-92. [Crossref] [PubMed]
- Lison S, Weiss G, Spannagl M, et al. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis 2011;22:190-6. [Crossref] [PubMed]
- Kon ZN, Brown EN, Tran R, et al. Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg 2008;135:367-75. [Crossref] [PubMed]
- Kiaii B, McClure RS, Stewart P, et al. Simultaneous integrated coronary artery revascularization with long-term angiographic follow-up. J Thorac Cardiovasc Surg 2008;136:702-8. [Crossref] [PubMed]
- Adams C, Burns DJ, Chu MW, et al. Single-stage hybrid coronary revascularization with long-term follow-up. Eur J Cardiothorac Surg 2014;45:438-42. [Crossref] [PubMed]
- de Cannière D, Jansens JL, Goldschmidt-Clermont P, et al. Combination of minimally invasive coronary bypass and percutaneous transluminal coronary angioplasty in the treatment of double-vessel coronary disease: two-year follow-up of a new hybrid procedure compared with "on-pump" double bypass grafting. Am Heart J 2001;142:563-70. [Crossref] [PubMed]
- Dieberg G, Smart NA, King N. Minimally invasive cardiac surgery: A systematic review and meta- analysis. Int J Cardiol 2016;223:554-60. [Crossref] [PubMed]
- Wang XW, Qu C, Huang C, et al. Minimally invasive direct coronary bypass compared with percutaneous coronary intervention for left anterior descending artery disease: a meta-analysis. J Cardiothorac Surg 2016;11:125. [Crossref] [PubMed]
- Benedetto U, Raja SG, Soliman RFB, et al. Minimally invasive direct coronary artery bypass improves late survival compared with drug-eluting stents in isolated proximal left anterior descending artery disease: a 10-year follow-up, single-center, propensity score analysis. J Thorac Cardiovasc Surg 2014;148:1316-1322. [Crossref] [PubMed]
- Hong SJ, Lim DS, Seo HS, et al. Percutaneous coronary intervention with drug-eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc Interv 2005;64:75-81. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Walther T, et al. Is chronic total coronary occlusion a risk factor for long-term outcome after minimally invasive bypass grafting of the left anterior descending artery? Ann Thorac Surg 2010;89:1496-501. [Crossref] [PubMed]
- Trehan N, Malhotra R, Mishra Y, et al. Comparison of ministernotomy with minithoracotomy regarding postoperative pain and internal mammary artery characteristics. Heart Surg Forum 2000;3:300-6. [PubMed]
- Abusamra R, Król M, Milewski K, et al. Short and long-term results of endoscopic atraumatic coronary artery off-pump bypass grafting in patients with left anterior descending artery stenosis. Cardiol J 2019; [Crossref] [PubMed]
- Endo Y, Nakamura Y, Kuroda M, et al. The Utility of a 3D Endoscope and Robot-Assisted System for MIDCAB. Ann Thorac Cardiovasc Surg 2019;25:200-204. [Crossref] [PubMed]
- Halkos ME, Liberman HA, Devireddy C, et al. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;147:179-85. [Crossref] [PubMed]
- Tarola CL, Al-Amodi HA, Balasubramanian S, et al. Ultrafast Track Robotic-Assisted Minimally Invasive Coronary Artery Surgical Revascularization. Innovations (Phila) 2017;12:346-50. [Crossref] [PubMed]
- Currie ME, Romsa J, Fox SA, et al. Long‐term angiographic follow‐up of robotic‐assisted coronary artery revascularization. Ann Thorac Surg 2012;93:1426-31. [Crossref] [PubMed]
- Giambruno V, Chu MW, Fox S, et al. Robotic-assisted coronary artery bypass surgery: an 18-year single-centre experience. Int J Med Robot 2018;14:e1891. [Crossref] [PubMed]
- Loulmet D, Carpentier A. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- Canale LS, Mick S, Mihaljevic T, et al. Robotically assisted totally endoscopic coronary artery bypass surgery. J Thorac Dis 2013;5:S641-9. [PubMed]
- Halkos ME, Ford L, Peterson D, et al. The impact of hybrid coronary revascularization on hospital costs and reimbursements. Ann Thorac Surg 2014;97:1610-5. [Crossref] [PubMed]
- Bonatti JO, Zimrin D, Lehr EJ, et al. Hybrid coronary revascularization using robotic totally endoscopic surgery: perioperative outcomes and 5-year results. Ann Thorac Surg 2012;94:1920-6. [Crossref] [PubMed]
- Hansson EC, Jidéus L, Åberg B, et al. Coronary artery bypass grafting-related bleeding complications in patients treated with ticagrelor or clopidogrel: a nationwide study. Eur Heart J. 2016;37:189-97. [Crossref] [PubMed]
- Halkos ME, Vassiliades TA, Douglas JS, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting for the treatment of multivessel coronary artery disease. Ann Thorac Surg 2011;92:1695-701. [Crossref] [PubMed]
- Shen L, Hu S, Wang H, et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-year follow-up results from a single institution. J Am Coll Cardiol 2013;61:2525-33. [Crossref] [PubMed]
- Gąsior M, Zembala MO, Tajstra MPOL-MIDES (HYBRID) Study Investigators, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1277-83. [Crossref] [PubMed]
- Tajstra M, Hrapkowicz T, Hawranek MPOL-MIDES Study Investigators, et al. Hybrid Coronary Revascularization in Selected Patients With Multivessel Disease 5-Year Clinical Outcomes of the Prospective Randomized Pilot Study. JACC Cardiovasc Interv 2016;9:1790-7. [Crossref] [PubMed]
- Ganyukov V, Kochergin N, Shilov A, et al. Randomized Clinical Trial of Surgical vs. Percutaneous vs. Hybrid Revascularization in Multivessel Coronary Artery Disease: Residual Myocardial Ischemia and Clinical Outcomes at One Year-Hybrid coronary REvascularization Versus Stenting or Surgery (HREVS). J Interv Cardiol 2020;2020:5458064. [Crossref] [PubMed]
- Esteves V, Oliveira MAP, Feitosa FS, et al. Late clinical outcomes of myocardial hybrid revascularization versus coronary artery bypass grafting for complex triple-vessel disease: Long-term follow-up of the randomized MERGING clinical trial. Catheter Cardiovasc Interv 2020; [Crossref] [PubMed]
- Harskamp RE, Bagai A, Halkos ME, et al. Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1,190 patients. Am Heart J 2014;167:585-92. [Crossref] [PubMed]
- Sardar P, Kundu A, Bischoff M, et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. Catheter Cardiovasc Interv 2018;91:203-12. [Crossref] [PubMed]
- Reynolds AC, King N. Hybrid coronary revascularization versus conventional coronary artery bypass grafting: Systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11941. [Crossref] [PubMed]
- Hu S, Li Q, Gao P, et al. Simultaneous hybrid revascularization versus off-pump coronary artery bypass for multivessel coronary artery disease. Ann Thorac Surg 2011;91:432-8. [Crossref] [PubMed]
- Bachinksy WB, Abdelsalam M, Boga G, et al. Comparative study of same sitting hybrid coronary artery revascularization versus off-pump coronary artery bypass in multivessel coronary artery disease. J Interv Cardiol 2012;25:460-8. [Crossref] [PubMed]
- Bagiella E. Hybrid Coronary Revascularization Trial. ClinicalTrials.gov Identifier: NCT03089398. Available online: https://clinicaltrials.gov/ct2/show/NCT03089398
- Lytle BW, Blackstone EH, Sabik JF, et al. The effect of bilateral internal thoracic artery grafting on survival during 20 postoperative years. Ann Thorac Surg 2004;78:2005-12. [Crossref] [PubMed]
- Taggart DP, Altman DG, Flather M, et al. Associations Between Adding a Radial Artery Graft to Single and Bilateral Internal Thoracic Artery Grafts and Outcomes: Insights From the Arterial Revascularization Trial. Circulation 2017;136:454-63. [Crossref] [PubMed]
- Gaudino M, Puskas JD, Di Franco A, et al. Three Arterial Grafts Improve Late Survival: A Meta-Analysis of Propensity-Matched Studies. Circulation 2017;135:1036-44. [Crossref] [PubMed]
- Habib RH, Dimitrova KR, Badour SA, et al. CABG versus PCI:Greater benefit in long-term outcomes with multiple arterial bypass grafting. J Am Coll Cardiol 2015;66:1417-27. [Crossref] [PubMed]
- Locker C, Schaff HV, Daly RC, et al. Multiple arterial grafts improve survival with coronary artery bypass graft surgery versus conventional coronary artery bypass grafting compared with percutaneous coronary interventions. J Thorac Cardiovasc Surg 2016;152:369-79.e4. [Crossref] [PubMed]
- Halkos ME, Rab ST, Vassiliades TA, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass for the treatment of left main coronary stenosis. Ann Thorac Surg 2011;92:2155-60. [Crossref] [PubMed]
- Taniwaki M, Windecker S, Zaugg S, et al. The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J 2015;36:2167-76. [Crossref] [PubMed]
- Farooq V, Serruys PW, Zhang Y, et al. Short-term and long-term clinical impact of stent thrombosis and graft occlusion in the SYNTAX trial at 5 years: Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery trial. J Am Coll Cardiol 2013;62:2360-2369. [Crossref] [PubMed]
- Borges JC, Lopes N, Soares PR, et al. Five-year follow-up of angiographic disease progression after medicine, angioplasty, or surgery. J Cardiothorac Surg 2010;5:91. [Crossref] [PubMed]
- Waters D, Craven TE, Lespérance J. Prognostic significance of progression of coronary atherosclerosis. Circulation 1993;87:1067-75. [Crossref] [PubMed]
- Messerli AW, Misumida N. Hybrid Coronary Revascularization 5 Years On: Is Clinical Equipoise Good Enough? JACC Cardiovasc Interv 2018;11:853-5. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Sgouropoulou S, et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J Thorac Cardiovasc Surg 2020; In press. [Crossref] [PubMed]
- Kikuchi K, Une D, Endo Y, et al. Minimally Invasive Coronary Artery Bypass Grating Using Bilateral In Situ Internal Thoracic Arteries. Ann Thorac Surg 2015;100:1082-4. [Crossref] [PubMed]
- Lemma M, Atanasiou T, Contino M. Minimally invasive cardiac surgery-coronary artery bypass graft. Multimed Man Cardiothorac Surg 2013;2013:mmt007. [Crossref] [PubMed]
- Balkhy HH, Kitahara H, Mitzman B, et al. Robotic totally endoscopic beating-heart bypass to the right coronary artery: first worldwide experience. Eur J Cardiothorac Surg 2020;57:529-34. [PubMed]
Cite this article as: Gadelkarim I, Borger MA, Davierwala PM. Hybrid coronary revascularization. AME Med J 2020;5:41.

