Ruijin robotic thoracic surgery: S1+2+3 segmentectomy of the left upper lobe
Clinical data
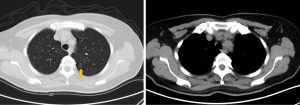
A 65-year-old woman was admitted because of pulmonary nodules for 1 month detected by computed tomography (CT). Chest CT (Figure 1) showed ground glass opacity (GGO) in the S1+2+3 segment of the left upper lobe. The patient’s complaints did not include chest tightness, shortness of breath, cough, expectoration, low fever, chills, night sweats or hoarseness. Cardiopulmonary function, blood gas analysis and laboratory tests were normal. There was no positive sign or supraclavicular lymph node enlargement in physical examination. She had no past medical history.
Operation steps
Anesthesia and body position
The patient received general anesthesia by double-lumen endotracheal intubation and was placed in the lateral decubitus position and in a jackknife position with single-lung (right) ventilation (Figure 2).
Ports
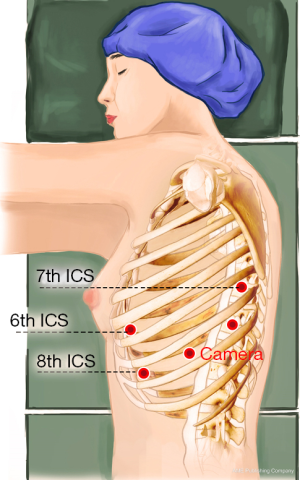
A 1.5-cm camera port (for a 12-mm trocar) was created in the 8th intercostal space (ICS) at the left mid axillary line, and three separate 1.0-cm working ports (for 8-mm trocars) were made in the 6th ICS (#1 arm) at the left anterior axillary line, the 8th ICS (#2 arm) at the left posterior axillary line, and the left 7th ICS (#3 arm), 2 cm from the spine. An auxiliary port (for a 12-mm trocar) was made in the 8th ICS near the costal arch (Figure 3).
Installation of the surgical arms
The robot patient cart was positioned above the operating table and then connected. The #1 arm was connected to a unipolar cautery hook and the #2 arm was connected with bipolar cautery forceps. An incision protector was used in the auxiliary port.
Surgical procedure
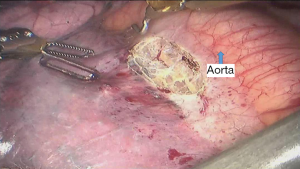
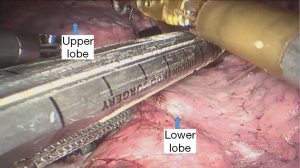
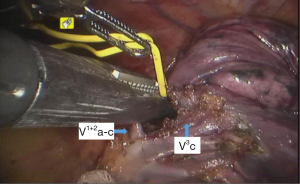
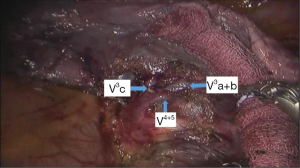
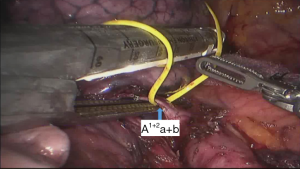
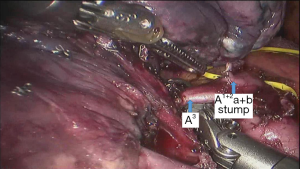
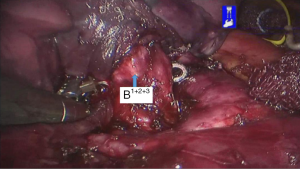
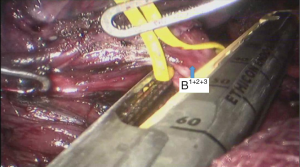
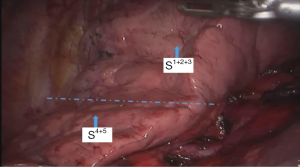
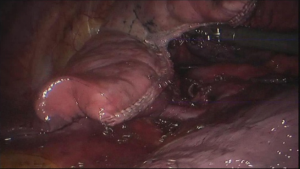
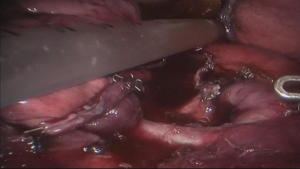
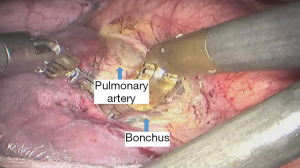
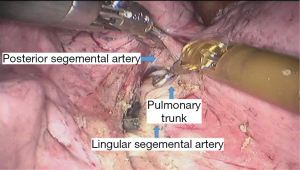
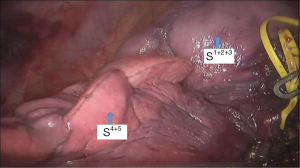
See Figures 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18.
Postoperative condition
Postoperative treatments included anti-inflammatory and phlegm-resolving treatments. The thoracic drainage tube was withdrawn 2 days after surgery, and the patient was discharged 3 days after surgery. No complications were observed during hospitalization. Pathological diagnosis was atypical adenomatous hyperplasia (AAH) at local alveolar epithelium of the S1+2+3 segment of the left upper lobe.
Discussion
The segmental dissection of the left upper lobe is a major challenge in robotic surgery because of the thin segmental vessels and bronchus. In addition, it is not easy to determine the segmental plane. The many arterial branches in the left upper lobe should be identified carefully when dissociating and pulling, especially the short branch A3. For that reason, the surgeon should be familiar with the fine anatomy of the vessels and bronchus. The robot has a clear and magnified field of view and flexible arms, which make dissection and use of Endo-GIA stapler easier compared with the thoracoscope. The #3 arm can help the surgeon to find the pulling location, which reduces the work of the assistant. In all surgeries, we dissect the hilum using a posterior approach and expose the pulmonary artery, which improves safety. We inject CO2 before removing the specimen of lung lobe to form a closed space, which keeps the operation field clear (1-5).
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.12). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhao X, Qian L, Lin H, et al. Robot-assisted lobectomy for non-small cell lung cancer in china: initial experience and techniques. J Thorac Dis 2010;2:26-8. [PubMed]
- Augustin F, Bodner J, Wykypiel H, et al. Initial experience with robotic lung lobectomy: report of two different approaches. Surg Endosc 2011;25:108-13. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
- Augustin F, Bodner J, Maier H, et al. Robotic-assisted minimally invasive vs. thoracoscopic lung lobectomy: comparison of perioperative results in a learning curve setting. Langenbecks Arch Surg 2013;398:895-901. [Crossref] [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
Cite this article as: Wu H, Yang S, Guo W, Jin R, Zhang Y, Chen X, Du H, Han D, Chen K, Xiang J, Li H. Ruijin robotic thoracic surgery: S1+2+3 segmentectomy of the left upper lobe. AME Med J 2017;2:2.