Robotic-assisted thoracoscopic surgery: right inferior lobectomy
Clinical data
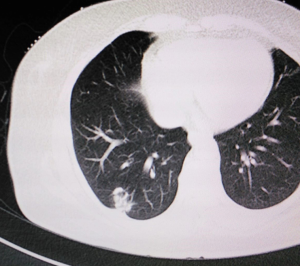
A 61-year old woman with chronic obstructive pulmonary disease but no history of smoking was found to have a right inferior lobe mass by computed tomography (CT) scan during health checkup. Adenocarcinoma was diagnosed by lung puncture. The right main and upper lobe bronchus were not involved. Positron emission tomography (PET)-CT and cerebral magnetic resonance imaging showed no distant metastasis. Preoperative blood analysis and tests of lung, cardiac, liver and renal function were normal. No superficial lymph node enlargement was detected on physical examination. The clinical stage was cT1N0M0. Informed consent for robotic-assisted thoracic lobectomy was obtained from patient before operation (Figure 1).
Procedure
Anesthesia and body position
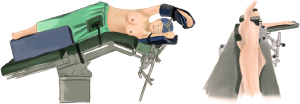
The patient received general anesthesia by double-lumen endotracheal intubation and was placed in the lateral decubitus position and in a jackknife position (Figure 2).
The port positions
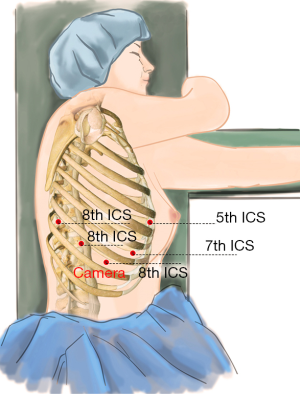
After the patient was prepped and draped in the usual manner, we placed five ports as follows: a 12-mm camera port was placed in the 8th intercostal space (ICS) at the mid axillary line, and three 10-mm working ports were placed separately in the 5th ICS at anterior axillary line (#1 arm), 8th ICS at the right posterior axillary line (#2 arm), and the right 8th ICS which was 2 cm from the spine (#3 arm). Finally, an auxiliary port was created in the 7th ICS between the camera port and the right working port, about 8-cm far from the right working port (Figure 3).
Connection of robot patient cart
The robot patient cart was positioned directly above the operating table. Two bipolar forceps and one unipolar cautery hook were attached to the arms. An incision protector was placed in the auxiliary port.
Surgical procedure
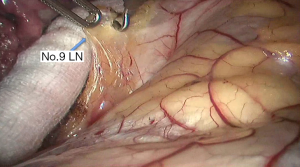
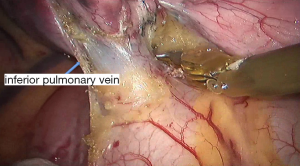
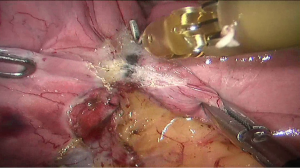
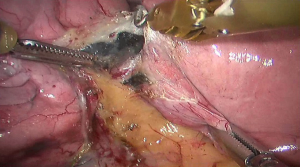
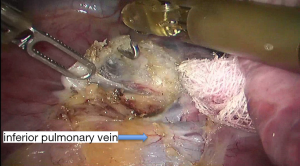
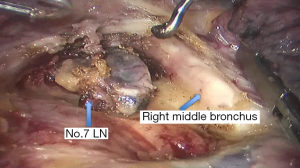
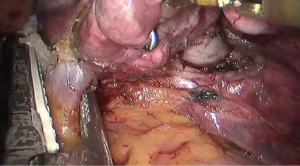
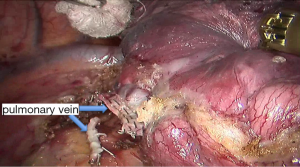
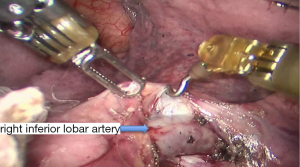
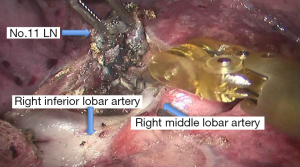
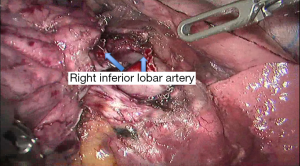
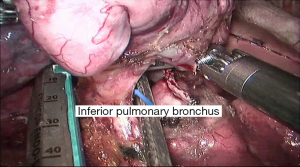
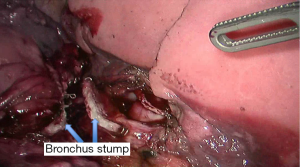
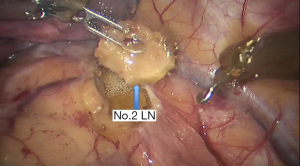
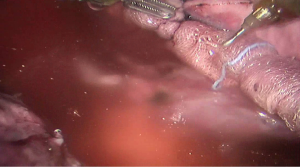
See Figures 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22.
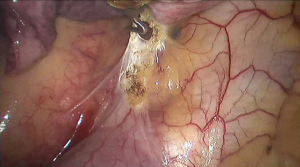
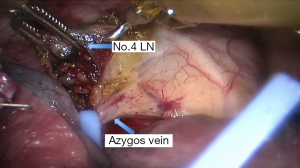
Postoperative outcome
The patient was routinely given anti-inflammatory and phlegm resolving treatment postoperatively. The chest tube was withdrawn after 2 days, and the patient was discharged 6 days later after surgery. No complications were occurred during hospitalization. The pathological stage was T1aN0M0 (stage IA).
Comment
Currently in our institution, the 3- or 4-arm method is mainly used. When the patient has a small physique, the 3-arm method may reduce interference between arms. However, the 4-arm method become more popular, because the visual field can be set at any angle in the thoracic cavity. Therefore, the position of each port is important. Nakamura et al. suggested a 9-cm distance should be set between ports to reduce interference between surgical arms (1). In our experience, a distance of 8–10 cm effectively reduces the interference between arms. Cerfolio et al. described a complete robotic lobectomy setting all the ports concentrating in the 7th ICS (2,3), and this method was considered applicable for all lobectomies (1).
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nakamura H, Taniguchi Y. Robot-assisted thoracoscopic surgery: current status and prospects. Gen Thorac Cardiovasc Surg 2013;61:127-32. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729-36; discussion 1736-7.
Cite this article as: Yang S, Guo W, Jin R, Zhang Y, Chen X, Wu H, Du H, Han D, Chen K, Xiang J, Li H. Robotic-assisted thoracoscopic surgery: right inferior lobectomy. AME Med J 2017;2:14.