Ruijin robotic thoracic surgery: robot-assisted enucleation of esophageal leiomyoma
Clinical data
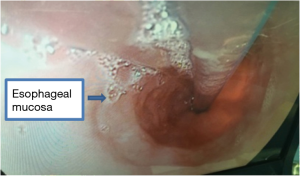
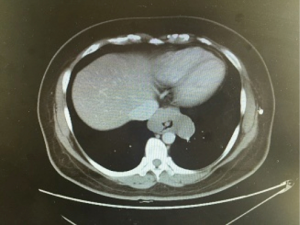
A 46-year-old asymptomatic and healthy woman was found incidentally to have a lower mediastinal mass on a screening X-ray. A computed tomography scan revealed a 6.6 cm × 4.2 cm homogeneous mass in the distal esophagus (Figure 1). A barium study demonstrated a filling defect of 6.4 cm in the distal esophagus. Esophagogastroduodenoscopy/endoscopic ultrasound was performed, demonstrating a partially obstructing submucosal mass 34 cm from the incisors with a normal overlying mucosa. The EUS demonstrated a hypoechoic and homogeneous mass in the fourth layer (muscularis propria) of the distal esophageal wall with no lymph node enlargement. Results of preoperative cardiopulmonary function and laboratory tests were normal. There was no positive sign on physical examination. She had no medical history.
Operation steps
Anesthesia and body position
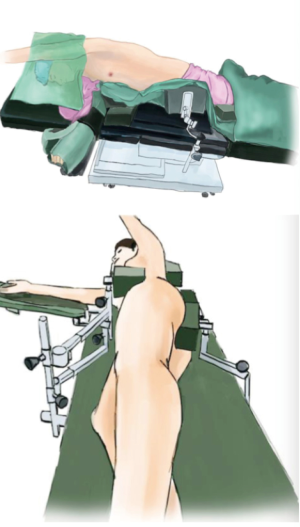
After the induction of general anesthesia, the patient was placed in a right lateral decubitus position under double-lumen endotracheal intubation. Her hands were placed in front of head, and she was placed in a Jackknife position with single-lung (right) ventilation (Figure 2).
Ports
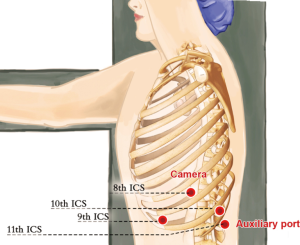
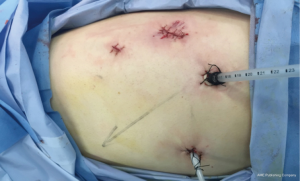
A 1.5-cm camera port (for a 12-mm trocar) was created in the 8th intercostal space (ICS) at left mid axillary line, and two 1.0-cm working ports (for 8-mm trocars) were made in the 10th ICS (#1 arm) at the left posterior axillary line and in the 9th ICS (#2 arm) at the left anterior axillary line. An auxiliary port (for a 12-mm trocar) was made in the 11th ICS at the left posterior axillary line (Figure 3).
Installation of the surgical arms
After all the trocars were positioned, the robot was positioned directly above the operating table and then connected. The #2 arm was connected to a bipolar cautery forceps and the #1 arm was connected to a unipolar cautery hook.
Surgical procedure
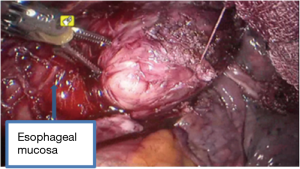
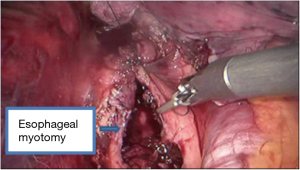
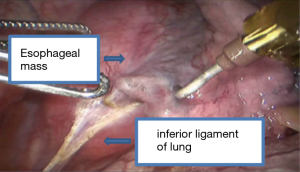
See Figures 4,5,6,7,8,9,10,11.
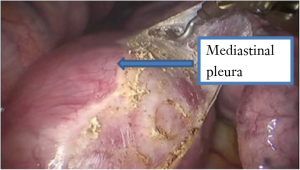
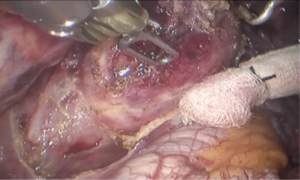
Postoperative condition
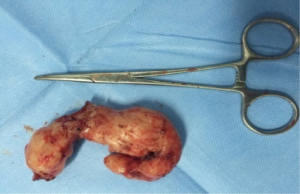
Postoperative care included anti-inflammatory, and phlegm-resolving treatments. The chest cavity drainage tube was withdrawn after 2 days, and the patient started a liquid diet. The patient was discharged on postoperative day 5 tolerating a semi-liquid diet. No complications were observed during hospitalization. Pathology confirmed an esophageal leiomyoma measuring 7 cm × 6 cm × 3 cm that was determined to be SMA, desmin and CD34-positive and CD117-, Ki67- and S-100-negative by immunohistochemistry.
Discussion
Leiomyoma, a rare esophageal neoplasm, is the most common benign esophageal neoplasm (1). Surgical resection is recommended in symptomatic case in which malignancy is suspected (2). The conventional treatment for esophageal leiomyoma is transthoracic enucleation by thoracotomy. However, open surgical approaches are associated with a high incidence of morbidities, significant postoperative pain, and long hospital stays. Over the years, minimally invasive surgery has more popular than conventional open thoracic surgery. However, these techniques have potential limitations. The angles and narrow spaces between the ribs may restrict movement, suturing, and dissection with thoracoscopy (3). Recently, robot-assisted thoracoscopic surgery using the da Vinci robot system has provided improved visualization and dexterity in esophageal procedures. The first case of robot-assisted enucleation of two esophageal leiomyomas (4.5 cm × 2.0 cm and 3.2 cm × 2.6 cm) was reported in 2004 (4). The thoracic esophagus is located deep in the mediastinum and surrounded by major organs including the heart, lungs, airway, and aorta. The robotic approach has advantages over thoracoscopic enucleation, providing extra degrees of freedom in a confined space. A more precise dissection is allowed by the wrist-like movement of the robotic instruments, the three-dimensional view, and magnification of images. There were some important details in the surgical enucleation of esophageal leiomyomas. Simultaneous intraoperative endoscopy was the key to success for the operation. It allowed the exact localization of the tumor and evaluated the integrity of the mucosa once the tumor was enucleated. Another important detail was the repair of the myotomy after the enucleation. Repair of the myotomy prevented mucosal bulging and possible formation of a diverticulum. The wrist-like movement of the robotic instruments can easily perform the suturing and knot tying.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seremetis MG, Lyons WS, deGuzman VC, et al. Leiomyomata of the esophagus. An analysis of 838 cases. Cancer 1976;38:2166-77. [Crossref] [PubMed]
- Nemir P Jr, Wallace HW, Fallahnejad M. Diagnosis and surgical management of benign diseases of the esophagus. Curr Probl Surg 1976;13:1-74. [Crossref] [PubMed]
- Kernstine KH, Andersen ES, Falabella A, et al. Robotic fourth-arm enucleation of an esophageal leiomyoma and review of literature. Innovations (Phila) 2009;4:354-7. [Crossref] [PubMed]
- Elli E, Espat NJ, Berger R, et al. Robotic-assisted thoracoscopic resection of esophageal leiomyoma. Surg Endosc 2004;18:713-6. [Crossref] [PubMed]
Cite this article as: Zhang Y, Yang S, Guo W, Jin R, Chen X, Wu H, Du H, Han D, Chen K, Xiang J, Li H. Ruijin robotic thoracic surgery: robot-assisted enucleation of esophageal leiomyoma. AME Med J 2017;2:16.