
Increased risk of diabetes in inflammatory orthopedics diseases
Introduction
Type 2 diabetes (T2DM) is a common metabolic disorder caused by insulin resistance (IR), obesity, physical inactivity and β-cell dysfunction (1,2). The prevalence of T2DM substantially increased in recent years. In Asian population, more than 110 million individuals were living with diabetes, with a disproportionate burden among the young and middle aged in 2007 (2). In 2010, the prevalence of T2DM had reached 11.6% in China, which indicating that 113.9 million people aged 18 years or older suffering from T2DM (3). The prevalence of the disease is expected to rise to 592 million by 2035 (4). T2DM leads to a heavy health burden on people and healthcare system (5,6).
T2DM was characterized by defect in insulin secretion and reduced response to insulin-stimulated glucose uptake and oxidative stress, glucotoxicity, lipotoxicity and endoplasmic reticulum stress all contributed to its pathogenesis (7,8). All of these mechanisms were related to inflammatory responses (9). In pancreatic islets, free fatty acids (FFA) was able to activate Toll-like receptor 2 (TLR2) and TLR4 and translocate nuclear factor κB (NF-κB) to release of inflammatory cytokines and chemokine such as tumor necrosis factor (TNF), interleukin-1β (IL-1β) and IL-8 (10). Also, increased demand for insulin was an induction of endoplasmic reticulum stress, which activated the inflammasome (11). Similar alterations have been observed in liver, muscle and other insulin-sensitive tissues. Inflammation was an established mechanism in the in the pathogenesis of T2DM and associated complications (4).
Chronic inflammatory diseases, such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), osteoarthritis (OA) and osteomyelitis, were common inflammatory conditions in orthopedics. In T2DM patients, 10–20% of patients with diabetes-related foot ulcers suffered from osteomyelitis (12). Various studies indicated that diabetes was able to increase the severity of inflammatory response in arthritis and osteomyelitis (13-18). However, only a few studies concern the risk of diabetes in patients suffering arthritis and osteomyelitis.
Here, we reviewed the risk of diabetes in different chronic inflammatory diseases in orthopedics and intended to raise the attention of higher risk of diabetes in various arthritis and osteomyelitis.
Risk of diabetes in inflammatory arthritis
Inflammatory arthritic diseases are a serious of autoimmune disorders where the host immune system invades self-defense mechanism and results in degeneration of the normal immune response and inflammation. RA, psoriatic arthritis (PsA) and AS are typical inflammatory arthritic diseases (19). RA is a major inflammatory disorder with the prevalence of 0.5–1% in the world population (20).
Risk of diabetes in RA
RA is associated with increased cardiovascular morbidity and mortality (21-23), which is resulted in the interactions among RA-related inflammatory activity, medications and traditional cardiovascular disease risk factors (24,25). Among these risk factors, T2DM is one of the most important (26,27).
In 2006, researchers enrolled 28,208 RA patients to compare the risk factors between patients and controls and found the prevalence ratio of T2DM was 1.4 in RA patients compared with controls (28). However, following studies investigating DM prevalence in RA had inconsistent results. Simard et al. examined the cross-sectional association between prevalent RA and diabetes among US civilians older than 60 years (n=5,302) but found no evidence of association between prevalent RA and diabetes in subjects aged over 60 years (29). With the help of electronic medical records database of the UK general population from 1986 to 2010, researchers found that the incidence rate for diabetes was 6.3 case per 1,000 person-years in patients with RA and the hazard ratio (HR) for diabetes after matched for age and sex was 1.12. However, the HR was attenuated after adjusting for age, sex, BMI, smoking and alcohol (14) and with further adjustment for baseline glucocorticoid (GC) use and co-morbidities, the HR was 0.94 (0.84–1.06).
Concerning the inconsistent results above, not only the inflammatory activity in RA, but also some RA medications could impact glucose metabolism, IR and consequently DM development.
GC is one of the most common medications in the treatment of RA due to its strong anti-inflammatory actions. However, GC can result in IR and hyperglycemia and in a duration-dependent and dose-dependent manner (30). Movahedi et al. assessed the risk of T2DM associated with the duration, dosage and time of GC usage in RA patients by using UK primary care database (CPRD) and US National Data Bank for Rheumatic Diseases (NDB). The HR was 1.30 and 1.61 in current users compared with nonusers in CPRD and NDB, respectively. The risk increased with GC dosage and duration but doses taken >6 months previously did not influence current risk (30). Ozen et al. conducted an investigation of the impact of DMARD and statin treatments on the incident of T2DM (31). By analyzing RA patients in NDB who were without T2DM at baseline and followed up from 2000 through 2014, researchers found that adjusted HR for DM were 1.31 for GC and 1.56 for statins while other synthetic/biological DMARDs were not associated with any risk change.
There are several mechanisms how GC increase the risk of diabetes. First, GCs can induce peripheral IR by impairing insulin signaling, which results in reduced glucose disposal and augmented endogenous glucose production. Second, GCs can promote abdominal obesity, elevate plasma fatty acids and triglycerides, and suppress osteocalcin synthesis in bone tissue. Third, pancreatic β-cells undergo several morphofunctional adaptations that result in hyperinsulinemia and failure of β-cells to compensate for this situation favors glucose homeostasis disruption, which can result in hyperglycemia (32).
Alternatively, hydroxychloroquine (HCQ) (33-35), methotrexate (MTX) (36) and TNF inhibitors (TNFi) (37,38) have been shown to favorably alter glucose metabolism. Large epidemiological studies showed decreased risk of new-onset DM with HCQ in RA (39,40). A total of 121,280 participants diagnosed with RA were enrolled to investigate the risk of newly recorded DM and multivariate adjusted analysis indicated the HR for T2DM were 0.62 for TNF inhibitors, 0.77 for MTX, and 0.54 for HCQ compared with other DMARDS (39). From the study of Ozen, the adjusted HR for DM was 0.67 for HCQ, 0.52 for abatacept (compared with MTX monotherapy). Also, concomitant use of GC did not alter DM risk reduction with HCQ (HR =0.69) (31). In patients with both RA and T2DM, the reduction in glycosylated hemoglobin (HbA1c) among patients taking HCQ was 0.54% greater than those taking MTX after fully adjusted analysis (36). Further studies support the potential benefit of HCQ in attenuating the risk of diabetes in RA patients (40,41).
HCQ reduced diabetes risk by improving insulin sensitivity and pancreatic β-cell functions (42,43), which may be independent of anti-inflammatory actions. In healthy individuals, the improvement in insulin sensitivity has been reported after HCQ treatment for 8 weeks (42,43). Furthermore, inflammatory cytokines in RA, particularly TNF-α and IL-6 were associated with IR and increased adiposity by triggering key steps in the insulin signaling pathways (44,45). In non-diabetic individuals, HCQ improved the adiponectin levels without significant change in serum inflammatory cytokines (TNF-α, IL-6) (43).
Apart from the risk of T2DM, RA is associated with increased risk of type 1 diabetes (T1DM). In a recent meta-analysis, the pooled risk estimate of 11 case-control studies showed a statistically significant increased risk of DM prevalence among RA individuals (T1DM, OR =4.87; T2DM, OR =1.41) (46). However, the mechanism underling the occurrence of T1DM in RA patients is different from that in T2DM. Both RA and T1DM are two autoimmune disorders that have been reported to co-occur in the same subjects and sharing of disease susceptibility loci is an accepted mechanism. Single-nucleotide polymorphisms such as SKAP2/rs7804356, GLIS3/rs7020673, PRKCQ/DKFZp667F0711/rs947474, GSDMB/rs2290400, BACH2/rs11755527, C6orf173/rs9388489, and DLK1/rs941576 are potential loci (47). Also, the involvement of oxidative stress may trigger genetically controlled autoimmunity to reactive oxygen species (ROS)—collagen type II (CII) and explain the association between T1DM and RA (48).
Risk of diabetes in psoriatic and psoriasis arthritis
Apart from RA, psoriatic (PsA) and psoriasis arthritis (PsO) were also common chronic, inflammatory arthritis. Nas et al. examined the association between PsA and its comorbid conditions. When compared with RA, PsA had relatively lower frequency of comorbidities like diabetes mellitus, hypertension and cataract/glaucoma surgery (49). Based on the CPRD database, researchers found that patients with severe PsO had significantly higher rates of diabetes (HR =1.23) compared with mild PsO (50). Radner et al. compared the incidence of cardiovascular risk factors of diabetes, hypertension, hyperlipidemia and obesity in patients with PsA, PsO and RA. The prevalence for diabetes was 6.2%, 6.3% and 7.8% in RA, PsO, and PsA, respectively. Incidence rates per 1,000 patient-years during follow-up for RA, PsO, and PsA cohorts for diabetes were 10.6, 13.0, and 14.7. The prevalence of hypertension, hyperlipidemia and obesity had similar trend in this cohort (51).
Risk of diabetes in AS
AS is a chronic inflammatory disease of the axial skeleton with approximately 0.2–0.9% of the general populations affected (52,53). Previous studies have suggested that patients with AS exhibit increased cardiovascular mortality and morbidity (54,55) and cerebrovascular diseases, such as stroke, were more common in patients with AS (28). IR and following diabetes may make contribution to it. However, the research concerning the relationship between AS and T2DM was few. Brophy et al. included 1,686 AS patients and 1,206,621 controls and found the prevalence of diabetes and hypertension, but not hyperlipidemia/hypercholesterolemia, were higher in AS (56). Using the Taiwan National Health Insurance data (57), researchers found that the risk of T2DM in the AS patients in Taiwan was 17.4% higher than that in the general population. Compared with non-AS cohort, the incidence of T2DM was 1.17-fold higher in the AS cohort (11.5 vs. 13.5, per 1,000 person-years), with an adjusted HR of 1.16 (58). Furthermore, the incidence of T2DM in women with AS was higher than that in men with AS (14.8 vs. 12.3 per 1,000 person-years).
Risk of diabetes in OA
OA is the most frequent musculoskeletal disease in subjects over 65 years old (59) and leads to functional decline and loss in quality of life (60). OA is characterized by joint pain, tenderness, stiffness, crepitus, limitation of movement, variable degrees of local inflammation and occasional effusion and in clinical practice (61). In a cross-sectional study of 543 primary care patients over 65 years old, 47.3% had OA and 14.2% had diabetes (62). The prevalence of OA in diabetes patients was 2-fold greater in those without diabetes, even after controlling for OA risk factors (63). A bundle of factors made contribution to OA pathophysiology in diabetes, including pro-inflammatory cytokines and adipokines, abnormal metabolites, acute phase proteins, vitamin D deficiency, and deregulated microRNAs (64). Diabetes was an independent risk factor for OA (65,66).
Despite the frequent occurrence of OA in people with diabetes, little is known about the impact of OA on the occurrence of diabetes and its complications. OA caused joint pain, stiffness, and reduced range of motion, which result in fatigue, depressed mood and functional limitations, including difficulty walking (67).
In people with hip and knee OA, difficulty walking has been independently linked with higher risk for all-cause death and cardiovascular events (68). Moreover, receipt of hip or knee joint replacement surgery reduced these risks (69), suggesting a direct relationship between difficulty walking and cardiovascular morbidity in patients with OA. However, the relationship between OA and the risk of diabetes was seldom investigated.
In Canadian Rheumatology Association 2017 Annual Meeting, Prof. Kendzerska presented the newest data on the relationship between OA and diabetes a population cohort. In this study, 16,362 subjects who were over 55 years old and absent of diabetes were enrolled from 1996–1998 and followed them to 2014. A total of 1,637 subjects suffered from OA in hip, 2,431 subjects suffered from OA in knee while there were 3,539 subjects developed into diabetes in the whole period of study. After adjusted for baseline age, gender, income, body mass index, history of hypertension and cardiovascular diseases, the risk for diabetes were significantly increased in subjects with OA. HR for OA in hip and knee were 1.25 and 1.16, respectively. However, this relationship disappeared after further adjusted for difficulty walking. Hence, researchers stated that the risk of diabetes was significantly increased in patients with OA, and this was mainly caused by the difficulty in walking.
Besides the study on the risk of diabetes in OA patients, the group also investigated the potential impact of OA-related pain and disability on diabetes outcomes. In the subjects with hip and knee OA from population cohort above (n=2,225), one in six reported a diabetes diagnosis. In subjects with both diabetes and OA, baseline difficulty walking was a predictor of risk for diabetes complications. Greater baseline difficulty walking was associated with shorter time to the first diabetes-specific complication (adjusted HR per unit increase in difficulty walking score was 1.24). After controlled for risk factors for diabetes complications, cardiovascular events and for other conditions that may affect mobility, those findings were still robust (70). OA-related difficulty walking exacerbated the accumulation of advanced glycation end products, dyslipidemia, hyperglycemia and systemic inflammation (71), and increasing risk for diabetes complications. Hence, researchers suggested that difficulty walking in people with diabetes may be a modifiable risk factor for complications.
Difficulty walking and accompanied obesity is an explanation for the increased risk of in OA (72). In the past few decades, the diabetes mellitus incidence have doubled, with an increasing number of T2DM cases (73). The level of FFA is elevated in obesity and FFA mediate many metabolic dysfunctions, especially IR (74). Elevated FFA is associated with both peripheral glucose uptake, hepatic gluconeogenesis and impaired glucose tolerance (75). Long-term lipid overload can result in the accumulation of lipids (such as triglyceride) in insulin responsive tissue and promote the lipotoxicity in cells (76).
Risk of diabetes in osteomyelitis
Chronic osteomyelitis (COM) is a chronic infection-inflammation status and is a common complication of T2DM (77). Osteomyelitis occurs in approximately 10–20% of patients with diabetes-related foot ulcers (12). Since chronic inflammation is a well-known risk factor for T2DM, COM is excepted to increase the risk of T2DM. However, only one study concerned the influence of COM on the risk of developing T2DM.
Using a retrospective cohort study from the Taiwan National Health Insurance Database (NHIRD) from 1997 to 2010, Lin et al. identified 20,641 patients with COM and 82,564 age- and sex-matched controls for comparison. The incidence of T2DM in COM patients was 1.6-fold higher than in controls (29.1 vs. 18.2 per 10,000 person-years) (78). The COM patients exhibited a higher diabetes risk (adjusted HR =1.64) after controlled the baseline and comorbidities. Also, an increased risk of T2DM in COM patients with comorbidities (adjusted HR =1.70) was observed when compared with that of their non-COM counterparts. Younger and higher income patients exhibited a higher COM-to-reference incidence rate ratio for T2DM compared with that of their counterparts.
COM increased the risk of T2DM via following methods. First, proinflammatory cytokines, such as TNF-α, IL-1β and IL-6 increased markedly in the bone compartment of COM patients (79), which could block downstream insulin signaling, interrupt insulin action and contribute to preclinical stages of T2DM (80). Second, COM patients were immobile because of pain or difficulty in engaging in physical activity [25], where the lack of physical activity could increase the risks of obesity, metabolic syndrome, and T2DM. Third, COM patients needed to make frequent medical visits due to the relapse of COM, where blood glucose levels were frequently tested and increased the likelihood to detect T2DM.
Conclusions
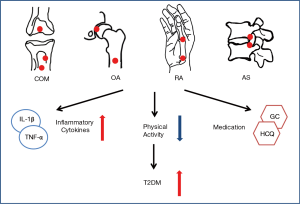
Patients with inflammation related arthritis and osteomyelitis had higher risk for diabetes in various long follow-up cohorts. The causes were as follows (Figure 1): (I) Local and systemic increased of inflammatory cytokines, such as TNF-α and IL-6, which triggered key steps in the insulin signaling pathways and resulted in increased adiposity and IR. (II) The pain or difficulty avoided activities such as walking and immobile status increased the risk of obesity, diabetes and metabolic syndrome. (III) The medical treatment of arthritis and osteomyelitis can influence the glycometabolism and insulin pathways. For example, GC and statins increased the risk of diabetes while HCQ and MTX were protective medication of diabetes. The combination of different treatment in various arthritis and osteomyelitis lead to different results in the occurrence of diabetes. In conclusion, inflammation related arthritis and osteomyelitis had higher risk of diabetes according to present limited studies. Further studies are needed to verify the conclusion and investigated the mechanism underlining the association between chronic orthopedics diseases and diabetes.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.05.25). Dr. Deng serves as an unpaid Section Editor of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taylor R. Type 2 diabetes: etiology and reversibility. Diabetes Care 2013;36:1047-55. [Crossref] [PubMed]
- Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009;301:2129-40. [Crossref] [PubMed]
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-59. [Crossref] [PubMed]
- Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 2014;13:465-76. [Crossref] [PubMed]
- Palermo A, Maggi D, Maurizi AR, et al. Prevention of type 2 diabetes mellitus: is it feasible? Diabetes Metab Res Rev 2014;30:4-12. [Crossref] [PubMed]
- Crandall JP, Knowler WC, Kahn SE, et al. The prevention of type 2 diabetes. Nat Clin Pract Endocrinol Metab 2008;4:382-93. [Crossref] [PubMed]
- Janikiewicz J, Hanzelka K, Kozinski K, et al. Islet beta-cell failure in type 2 diabetes--Within the network of toxic lipids. Biochem Biophys Res Commun 2015;460:491-6. [Crossref] [PubMed]
- Robertson RP, Harmon J, Tran PO, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53:S119-24. [Crossref] [PubMed]
- Hotamisligil GS, Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat Rev Immunol 2008;8:923-34. [Crossref] [PubMed]
- Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 2012;18:1279-85. [Crossref] [PubMed]
- Oslowski CM, Hara T, O'Sullivan-Murphy B, et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab 2012;16:265-73. [Crossref] [PubMed]
- Game F. Management of osteomyelitis of the foot in diabetes mellitus. Nat Rev Endocrinol 2010;6:43-7. [Crossref] [PubMed]
- Listing J, Gerhold K, Zink A. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology (Oxford) 2013;52:53-61. [Crossref] [PubMed]
- Dubreuil M, Rho YH, Man A, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology (Oxford) 2014;53:346-52. [Crossref] [PubMed]
- Ruscitti P, Cipriani P, Di Benedetto P, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1beta via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol 2015;182:35-44. [Crossref] [PubMed]
- Chen HH, Chen DY, Lin SY, et al. Periodontitis exposure within one year before anti-diabetic treatment and the risk of rheumatoid arthritis in diabetes mellitus patients: a population-based cohort study. Rev Bras Reumatol 2014;54:124-30. [Crossref] [PubMed]
- King KB, Rosenthal AK. The adverse effects of diabetes on osteoarthritis: update on clinical evidence and molecular mechanisms. Osteoarthritis Cartilage 2015;23:841-50. [Crossref] [PubMed]
- Laiguillon MC, Courties A, Houard X, et al. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthritis Cartilage 2015;23:1513-22. [Crossref] [PubMed]
- Albrecht S, Unwin L, Muniyappa M, et al. Glycosylation as a marker for inflammatory arthritis. Cancer Biomark 2014;14:17-28. [Crossref] [PubMed]
- Kirwan JR, Boers M. Biological treatment in rheumatoid arthritis: when to stop? Lancet 2014;383:288-9. [Crossref] [PubMed]
- Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524-9. [Crossref] [PubMed]
- Meune C, Touze E, Trinquart L, et al. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford) 2009;48:1309-13. [Crossref] [PubMed]
- Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003;107:1303-7. [Crossref] [PubMed]
- Gonzalez A, Maradit Kremers H, Crowson CS, et al. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheum Dis 2008;67:64-9. [Crossref] [PubMed]
- Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis 2010;69:1920-5. [Crossref] [PubMed]
- Dinesh Shah A, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet 2015;385:S86. [Crossref] [PubMed]
- Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol 2015;3:105-13. [Crossref] [PubMed]
- Han C, Robinson DW Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2006;33:2167-72. [PubMed]
- Simard JF, Mittleman MA. Prevalent rheumatoid arthritis and diabetes among NHANES III participants aged 60 and older. J Rheumatol 2007;34:469-73. [PubMed]
- Movahedi M, Beauchamp ME, Abrahamowicz M, et al. Risk of Incident Diabetes Mellitus Associated With the Dosage and Duration of Oral Glucocorticoid Therapy in Patients With Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1089-98. [PubMed]
- Ozen G, Pedro S, Holmqvist ME, et al. Risk of diabetes mellitus associated with disease-modifying antirheumatic drugs and statins in rheumatoid arthritis. Ann Rheum Dis 2017;76:848-54. [PubMed]
- Rafacho A, Ortsater H, Nadal A, et al. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol 2014;223:R49-62. [Crossref] [PubMed]
- Penn SK, Kao AH, Schott LL, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol 2010;37:1136-42. [Crossref] [PubMed]
- Solomon DH, Garg R, Lu B, et al. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: a randomized, blinded crossover trial. Arthritis Care Res (Hoboken) 2014;66:1246-51. [Crossref] [PubMed]
- Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken) 2014;66:1619-26. [Crossref] [PubMed]
- Rekedal LR, Massarotti E, Garg R, et al. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum 2010;62:3569-73. [Crossref] [PubMed]
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol 2006;24:83-6. [PubMed]
- Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, et al. Insulin resistance in rheumatoid arthritis: the impact of the anti-TNF-alpha therapy. Ann N Y Acad Sci 2010;1193:153-9. [Crossref] [PubMed]
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 2011;305:2525-31. [Crossref] [PubMed]
- Wasko MC, Hubert HB, Lingala VB, et al. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA 2007;298:187-93. [Crossref] [PubMed]
- Bili A, Sartorius JA, Kirchner HL, et al. Hydroxychloroquine use and decreased risk of diabetes in rheumatoid arthritis patients. J Clin Rheumatol 2011;17:115-20. [Crossref] [PubMed]
- Mercer E, Rekedal L, Garg R, et al. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res Ther 2012;14:R135. [Crossref] [PubMed]
- Wasko MC, McClure CK, Kelsey SF, et al. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: a randomised trial. Diabetologia 2015;58:2336-43. [Crossref] [PubMed]
- Chung CP, Oeser A, Solus JF, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum 2008;58:2105-12. [Crossref] [PubMed]
- Tam LS, Tomlinson B, Chu TT, et al. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol 2007;26:1495-8. [Crossref] [PubMed]
- Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol 2015;33:115-21. [PubMed]
- Kiani AK, Jahangir S, John P, et al. Genetic link of type 1 diabetes susceptibility loci with rheumatoid arthritis in Pakistani patients. Immunogenetics 2015;67:277-82. [Crossref] [PubMed]
- Strollo R, Rizzo P, Spoletini M, et al. HLA-dependent autoantibodies against post-translationally modified collagen type II in type 1 diabetes mellitus. Diabetologia 2013;56:563-72. [Crossref] [PubMed]
- Nas K, Karkucak M, Durmus B, et al. Comorbidities in patients with psoriatic arthritis: a comparison with rheumatoid arthritis and psoriasis. Int J Rheum Dis 2015;18:873-9. [Crossref] [PubMed]
- Edson-Heredia E, Zhu B, Lefevre C, et al. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using Clinical Practice Research Datalink. J Eur Acad Dermatol Venereol 2015;29:955-63. [Crossref] [PubMed]
- Radner H, Lesperance T, Accortt NA, et al. Incidence and Prevalence of Cardiovascular Risk Factors Among Patients With Rheumatoid Arthritis, Psoriasis, or Psoriatic Arthritis. Arthritis Care Res (Hoboken) 2016.
- Gran JT, Husby G. Clinical, epidemiologic, and therapeutic aspects of ankylosing spondylitis. Curr Opin Rheumatol 1998;10:292-8. [Crossref] [PubMed]
- Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl 2006;78:4-11. [PubMed]
- Radford EP, Doll R, Smith PG. Mortality among patients with ankylosing spondylitis not given X-ray therapy. N Engl J Med 1977;297:572-6. [Crossref] [PubMed]
- Mathieu S, Gossec L, Dougados M, et al. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:557-63. [Crossref] [PubMed]
- Brophy S, Cooksey R, Atkinson M, et al. No increased rate of acute myocardial infarction or stroke among patients with ankylosing spondylitis-a retrospective cohort study using routine data. Semin Arthritis Rheum 2012;42:140-5. [Crossref] [PubMed]
- Jiang YD, Chang CH, Tai TY, et al. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000-2009 Nationwide Health Insurance database. J Formos Med Assoc 2012;111:599-604. [Crossref] [PubMed]
- Chen HH, Yeh SY, Chen HY, et al. Ankylosing spondylitis and other inflammatory spondyloarthritis increase the risk of developing type 2 diabetes in an Asian population. Rheumatol Int 2014;34:265-70. [Crossref] [PubMed]
- McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363-88. [Crossref] [PubMed]
- Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port 2015;28:99-106. [Crossref] [PubMed]
- Woolf AD. Driving musculoskeletal health for Europe: EUMUSC.NET. Reumatismo 2011;63:1-4. [Crossref] [PubMed]
- Boeckxstaens P, Peersman W, Goubin G, et al. A practice-based analysis of combinations of diseases in patients aged 65 or older in primary care. BMC Fam Pract 2014;15:159. [Crossref] [PubMed]
- Nieves-Plaza M, Castro-Santana LE, Font YM, et al. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. J Clin Rheumatol 2013;19:1-6. [Crossref] [PubMed]
- Wang X, Hunter D, Xu J, et al. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage 2015;23:22-30. [Crossref] [PubMed]
- Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract 2016;122:198-206. [Crossref] [PubMed]
- Schett G, Kleyer A, Perricone C, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care 2013;36:403-9. [Crossref] [PubMed]
- Hawker GA, Gignac MA, Badley E, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1382-90. [Crossref] [PubMed]
- Hawker GA, Croxford R, Bierman AS, et al. All-cause mortality and serious cardiovascular events in people with hip and knee osteoarthritis: a population based cohort study. PLoS One 2014;9:e91286. [Crossref] [PubMed]
- Ravi B, Croxford R, Austin PC, et al. The relation between total joint arthroplasty and risk for serious cardiovascular events in patients with moderate-severe osteoarthritis: propensity score matched landmark analysis. BMJ 2013;347:f6187. [Crossref] [PubMed]
- Hawker GA, Croxford R, Bierman AS, et al. Osteoarthritis-related difficulty walking and risk for diabetes complications. Osteoarthritis Cartilage 2017;25:67-75. [Crossref] [PubMed]
- Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA 2013;310:1263-73. [Crossref] [PubMed]
- De Feo P, Boris JM, Maffeis C. Lifestyle modification strategies to counteract the world epidemic growth of obesity and diabetes. Biomed Res Int 2014;2014:640409.
- Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 2016;118:1723-35. [Crossref] [PubMed]
- Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 2011;60:2441-9. [Crossref] [PubMed]
- Staehr P, Hothernielsen O, Landau BR, et al. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 2003;52:260. [Crossref] [PubMed]
- Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 1995;44:863-70. [Crossref] [PubMed]
- Hatzenbuehler J, Pulling TJ. Diagnosis and management of osteomyelitis. Am Fam Physician 2011;84:1027-33. [PubMed]
- Lin SY, Lin CL, Tseng CH, et al. The association between chronic osteomyelitis and increased risk of diabetes mellitus: a population-based cohort study. Eur J Clin Microbiol Infect Dis 2014;33:1647-52. [Crossref] [PubMed]
- Fullilove S, Jellis J, Hughes SP, et al. Local and systemic concentrations of tumour necrosis factor-alpha, interleukin-6 and interleukin-8 in bacterial osteomyelitis. Trans R Soc Trop Med Hyg 2000;94:221-4. [Crossref] [PubMed]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860-7. [Crossref] [PubMed]
Cite this article as: Jin J, Wang Y, Deng Z. Increased risk of diabetes in inflammatory orthopedics diseases. AME Med J 2017;2:71.

