Is it safe to consider anticoagulation in cirrhotic patients with venous thromboembolism?
Venous thromboembolism (VTE) increases the morbidity and mortality of the hospitalized patients. VTE can be seen in two different groups of people admitted to the hospitals. One group of patients is at very high risk of bleeding and the second group of patients is those without or with minimal risk of bleeding. When we plan thromboprophylaxis or treatment for VTE with the anticoagulants we are concerned about the risk of bleeding in special patients. Therefore, one should be aware of the number needed to harm (NNH) while considering the anticoagulation. In one population-based cohort of Coumadin patients 2% experienced a fatal bleeding event (NNH =50) and the overall major bleeding rate was 12% (NNH =8) (1). It was estimated that 15,000–52,000 patients die annually in the US of brain bleeding caused by Coumadin. Incidence of VTE in the general population is found to be 1–2 per 1,000 person-years. But it can be as high as 1 per 100 person-years in patients with co-morbidities such as heart failure, cardiovascular diseases and chronic liver diseases (CLD) (2). The recent literature shows that CLD leads to an imbalance in the coagulation system. It was shown that the liver disease-induced coagulopathy is also associated with thrombosis (3-5). Several previous studies had reported a varying incidence of VTE rate among cirrhotic patients ranging from 0.5% to 6.3% (6-10). Hemostasis is a well-balanced physiologic process and it is significantly altered in cirrhosis. The cumulative effect of coagulation dysregulation in CLD is complex, leading to both hemorrhagic and thrombotic complications. In cirrhotic patient elevation of INR occurs due to a reduction in the synthesis of procoagulant factors II, V, VII, X, XI, XII, XIII and fibrinogen, which contributes to the increased bleeding risk. Cirrhosis is also a disease state associated with hypercoagulability due to decreased synthesis of anticoagulant factors, such as anti-thrombin, proteins C and S, as well as decreased production of fibrinolytics such as plasminogen. The recent observations on hemostasis in patients with CLD challenge the dogma that the major coagulopathy in these patients leads consistently to bleeding. These changes that accompany CLD may restore the balance of anticoagulant and procoagulant effects. In certain circumstances, the risk of thrombotic events may be greater than the risk of hemorrhage. We are realizing that anticoagulants are often regarded contraindicated in patients with CLD, but they may instead prove beneficial and they should be tested in appropriate clinical trials (11,12).
There are five independent risk factors for major bleeding: (I) age 65 years or greater; (II) history of stroke; (III) history of gastrointestinal bleeding; (IV) a serious co-morbid condition (recent myocardial infarction, renal insufficiency, or severe anemia); (V) atrial fibrillation—predicted major bleeding in the testing group; the cumulative incidence of major bleeding at 48 months was 2% in 57 low-risk patients, 17% in 110 middle-risk patients, and 63% in 20 high-risk patients (1). In 1990, Landefeld et al. said that the bleeding risk index provides valid estimates of the probability of major bleeding in hospitalized patients starting long-term anticoagulant therapy and complements physicians’ predictions. The possibility that bleeding can be prevented in high-risk patients warrants prospective evaluation. But they have also included the liver dysfunction as one of the risk factors for the bleeding with anticoagulation (13). Anticoagulant-related bleeding is common and it can be serious. The risk for bleeding can be estimated in an individual patient, giving the primary physician a quantitative basis for weighing the risks and benefits of therapy and for optimizing patient management. The frequency of anticoagulant-related bleeding is reduced by less intense warfarin therapy. Future studies should evaluate new approaches to management that may further reduce complications while maintaining efficacy. The risk of bleeding associated with intravenous heparin therapy in patients with acute VTE is <3% in recent trials. There is some evidence to suggest that this bleeding risk increases with the heparin dosage and age (>70 years). LMW heparin is not associated with increased major bleeding compared with standard heparin in acute VTE. Standard heparin and LMW heparin are not associated with an increase in major bleeding in ischemic coronary syndromes, but are associated with an increase in major bleeding in ischemic stroke (14). Traditionally, liver cirrhosis has been considered as a disease with hypocoagulability state and increasing bleeding tendency due to severe homeostatic disruption in liver now there is increasing awareness and evidence to say that cirrhotic patients are not completely protected from thrombotic events although they have an elevated international normalized ratio and auto-anticoagulation. Hypercoagulability is an increasingly recognized aspect of CLD but the bleeding risk of VTE prophylaxis and treatment remains unclear. Sufficient evidence exists for considering the use of anticoagulation in cirrhotic patients for both VTE prophylaxis and treatment in selected patients after consideration of endoscopic variceal bleeding prophylaxis.
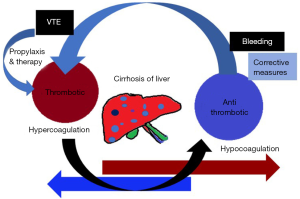
Hemostasis is a delicately balanced physiologic process that is significantly altered in cirrhosis (Figure 1). The cumulative effect of coagulation dysregulation in CLD is complex, leading to both hemorrhagic and thrombotic complications (15). In the general population, the incidence rate of VTE is 1–2 per 1,000 person-years. Among the elderly, cancer patients, or patients with multiple medical co-morbidities, such as chronic heart or lung disease, the incidence rate can be as high as 1 per 100 person-years. Moreover, an elevated partial thromboplastin time (PTT) and INR do not appear to protect them against the development of hospital-acquired VTE in CLD. In-hospital VTE was 21% higher in patients with compensated cirrhosis and 39% higher in patients with de-compensated cirrhosis, compared to hospitalized patients without liver disease up to the age of 45 and thereafter, the prevalence of VTE in non-liver disease patients was similar to the cirrhotic patients and this gap decreased with increasing age (16,17). However, patients with cirrhosis are usually excluded from the thrombosis trials and so, there is controversy over whether thromboprophylaxis would expose cirrhotic patients to an increased risk of bleeding with subsequently greater risk of morbidity and mortality is not resolved. So, it is less certain if this benefit extends to patients with cirrhosis. Expert opinions advocated that hospitalized cirrhotic patients should be considered for venous thromboprophylaxis (18).
A recent study found that 76% of inpatient cirrhotic patients received neither pharmacological nor mechanical DVT prophylaxis (10). Another group found that only 9% of hospitalized patients with CLD were treated with pharmacological VTE prophylaxis, while only 16% received mechanical VTE prophylaxis (5). At present, there are no large prospective randomized controlled trials evaluating the safety of VTE prophylaxis in cirrhosis. Northup et al. showed that the presence of esophageal varices was the only statistically significant independent risk factor for increased risk of bleeding. The other risk factors including model for end-stage liver disease (MELD) score, platelet count and age were not associated with increased risk for either VTE or bleeding risk (19). Bechmann et al. evaluated the safety of prophylactic anticoagulation in preventing VTE in hospitalized cirrhotic patients and found that prophylaxis was not associated with increased risk of bleeding or death (20). A study by Villa et al. (21) provides compelling data regarding the potential safety and efficacy of prophylactic anticoagulation in reducing the rate of acute portal vein thrombosis (PVT) in cirrhotic non-hospitalized patients. This study examined the use of enoxaparin in preventing PVT in patients with advanced stages of cirrhosis given Child-Pugh scores of B and C. The primary end point was portal vein or mesenteric vein thrombosis, and secondary end points included overall survival and clinical hepatic decompensation. These authors also examined the rate of hemorrhage and thrombocytopenia as safety end points related to anticoagulation. The study period was 24 weeks, in which enoxaparin 4,000 IU or placebo were administered daily for participants for 12 months, followed by 12-month observation period. No hemorrhagic events were reported at the end of the observation period (24 months), although one patient developed thrombocytopenia secondary to the enoxaparin.
New special tests are introduced for estimating the bleeding tendency in CLD. These include thrombin generation assay, thromboelastography (TEG) and thromboelastometry. The thrombin generation assay is modified by adding thrombomodulin to measure thrombin formation and thus, it can assess the serum coagulation factors and the coagulation inhibitors in patients with CLD. TEG measures the global homeostatic function including the initial interaction of platelets with fibrin, platelets aggregation through clot formation and clot dissolution. TEG has been shown to have better predictive capability of postoperative bleeding compared to conventional coagulation testing in cirrhotic patients (22).
In the February 2017 issue, Zhang et al. observed that the anticoagulation did not influence the risk of major bleeding and in-hospital death in cirrhotic patients with VTE, in a case-control study. It is difficult to pool data of a large number of cirrhotic liver disease patients with venous thrombosis from just one or two hospitals. They have arrived at a meaningful conclusion and it was corresponding with the findings from the other studies in the field. They also derived that the in-hospital mortality was less in the patients with cirrhosis and VTE when they received anticoagulation than when they did not receive the anticoagulation. So, they are questioning the role of “wait-and-see” policy in management of VTE in cirrhosis patients. There are also limitations to this study. It is a small study and there were only 16 study patients, their details of anticoagulation methods are not available. These results from a single center in a retrospective study need to be supported by prospective multicenter studies to establish the role of adequate anticoagulation for sufficient duration in the management of VTE in liver cirrhosis patients.
In summary, one can say that there is increasing evidence to promote the use of anticoagulation in cirrhotic patients for both VTE prophylaxis and treatment in selected patients after endoscopic variceal bleeding prophylaxis balancing the risks and benefits of anticoagulation.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037amj.2017.04.12). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med 1989;87:144-52. [Crossref] [PubMed]
- Heit J A. The epidemiology of venous thromboembolism in the community, implications for prevention and management. J Thromb Thrombolysis 2006;21:23-29. [Crossref] [PubMed]
- Gulley D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci 2008;53:3012-7. [Crossref] [PubMed]
- Dabbagh O, Oza A, Prakash S, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest 2010;137:1145-9. [Crossref] [PubMed]
- Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol 2006;101:1524-8;quiz 1680.
- García-Fuster MJ, Abdilla N, Fabiá MJ, et al. Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig 2008;100:259-62. [PubMed]
- Anthony Lizarraga W, Dalia S, Reinert SE, et al. Venous thrombosis in patients with chronic liver disease. Blood Coagul Fibrinolysis 2010;21:431-5. [Crossref] [PubMed]
- Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol 2010;8:800-5. [Crossref] [PubMed]
- Saleh T, Matta F, Alali F, et al. Venous thromboembolism with chronic liver disease. Am J Med 2011;124:64-8. [Crossref] [PubMed]
- Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J 2011;9:1. [Crossref] [PubMed]
- Ali M, Ananthakrishnan AN, McGinley EL, et al. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci 2011;56:2152-9. [Crossref] [PubMed]
- Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147-56. [Crossref] [PubMed]
- Landefeld CS, McGuire E 3rd, Rosenblatt MW. A bleeding risk index for estimating the probability of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med 1990;89:569-78. [Crossref] [PubMed]
- Levine MN, Raskob G, Landefeld S, et al. Hemorrhagic complications of anticoagulant treatment. Chest 2001;119:108S-21S. [Crossref] [PubMed]
- Khoury T, Ayman AR, Cohen J, et al. The Complex Role of Anticoagulation in Cirrhosis: An Updated Review of Where We Are and Where We Are Going. Digestion 2016;93:149-59. [Crossref] [PubMed]
- Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol 2010;25:116-21. [Crossref] [PubMed]
- Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009;51:682-9. [Crossref] [PubMed]
- Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691-7. [Crossref] [PubMed]
- Kanaan AO, Silva MA, Donovan JL, et al. Meta-analysis of venous thromboembolism prophylaxis in medically Ill patients. Clin Ther 2007;29:2395-405. [Crossref] [PubMed]
- Bechmann LP, Sichau M, Wichert M, et al. Low-molecular-weight heparin in patients with advanced cirrhosis. Liver Int 2011;31:75-82. [Crossref] [PubMed]
- Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253-60.e1-4.
- Mallett SV, Cox DJ. Thrombelastography. Br J Anaesth 1992;69:307-13. [Crossref] [PubMed]
Cite this article as: Pinjala RK. Is it safe to consider anticoagulation in cirrhotic patients with venous thromboembolism? AME Med J 2017;2:77.