Health benefits of soy and soy phytochemicals
Introduction
Soy (Glycine max) is an important commodity in the world’s food supply and economy (1). Soy consumption as a food has a long history in Asian countries, such as China and Korea (2), while the U. S. accounted for more than 30% of world production in 2013 (3). The popularity of soy foods in the west increased significantly after a 1999 decision by the U.S. Food and Drug Administration to allow health claim of soy protein’s protective effect against heart disease (4). The U.S. retail soy food industry grew from $1 billion in 1996 to $4.5 billion in 2013 (5). The nutritional/health promoting value of soy and soy food was the major contributing factor for the increasing demand, as 26% of U.S. consumers indicated that they are interested in soy foods specifically for the reported health benefits (6-8). Therefore, science-based information on health-promoting effects of soy and soy phytochemicals is of great economic and societal importance and can further promote the use of soy and soy phytochemicals.
Health benefits of soy
Protection against cardiovascular disease
Epidemiological studies have long linked soy consumption to a reduced risk for cardiovascular disease, and soy protein, soy-derived isoflavones, and soy sterols have been studied as the active components contributing to such effects (2,9-12). A recent review identified soy proteins to be partially responsible for the lipid-lowering effects of soy (10). It has also been suggested that isoflavones may act on the vascular tissue to improve circulation (12-14).
Soy’s cardiovascular protective effects were reviewed and highlighted in a meta-analysis of 38 controlled clinical trials in human subjects and the correlation between consumption of soy and lipid levels (15). It was concluded that an average consumption of 47 g soy protein/day resulted in significant decreases in total cholesterol (9.3%), LDL cholesterol (12.9%) and triglycerides (10.5%). On the other hand, in a more recent analysis, the Nutrition Committee of American Heart Association found a much smaller decrease in LDL (3%) with 50 g soy protein consumption per day from eight randomized trials and no apparent benefit in 14 other studies (9). The responses appeared to be highly dependent on the cholesterol level of the subjects. The meta-analysis performed by Anderson and colleagues recruited patients who were strongly hyperlipidemic (total cholesterol >250 mg/dL). It was expected that extreme hyperlipidemia would benefit from greater percentage reductions in total and LDL cholesterol. In contrast, the patients with moderate or minor hyperlipidemia recruited in the later studies showed less response to the soy protein.
One proposed mechanism of soy’s cardio-protective effects results from the inhibition of LDL oxidation and reduction of the formation of plaque in the arteries. Soy extracts (16,17) and peptides (18,19) have both been shown to reduce the oxidation of LDL cholesterol in vitro and in vivo. It was also reported that the anthocyanins found in color seed coat of soy displayed potent anti-oxidant properties and could inhibit LDL oxidation (16,20,21). A more recent hypothesis involves the liver’s LDL receptors, which may be affected by soy consumption to enhance their uptake of LDL from the serum and consequently lower serum LDL levels (22).
Protection against obesity-related metabolic syndrome
The potential of soy consumption in mitigating obesity and related complications were reported in recent studies (23,24). The exact efficacy is still under investigation. Mechanistically, the estrogen-like structures of soy isoflavones may contribute to similar activities, such as regulating adipogenesis by binding to estrogen receptors, thus decreasing lipoprotein lipase activity, and PPAR may also be involved in the regulation (24). Levels of genistein, a soy isoflavone, ranging from 0.1 to 1.0 µM have been shown to inhibit adipogenesis, while higher concentrations (25–50 µM) enhanced adipogenesis, showing a biphasic effect (24). However, evidence in animal models and in vitro study did not always apply well to human, which made it inconclusive whether consumption of soy will benefit the obese population. Human clinical experiments did not show any decrease in body weight as expected, but improvements in blood lipids (24) or improvements in insulin resistance was observed (25).
Protection against cancer
Soy has also shown potential in reducing the risk of many types of cancer (26-29) and, among which, breast and prostate cancers are of particular interest due to their sensitivity to sex steroid hormones.
Despite the amount of effort from experimental and epidemiological studies, soy products’ effect in breast and prostate cancer prevention or therapy is largely unresolved. Epidemiological studies among Asian population had long connected the lower breast cancer incident with consumption of soy products, which was usually attributed to soy isoflavones (30,31). Alongside the modest protective effects, concern has also been expressed that the estrogenic activity of soy isoflavones may lead to adverse effects on progression and recurrence of breast cancer (32). On the other hand, there were some reviews citing a number of human clinical and epidemiological studies indicating no evidence of risk in human (33-35). Preventative effect of soy against prostate cancer have been reviewed previously (36) and clinical trials have also shown soy’s therapeutic effect against prostate cancer (37,38), which, again, was challenged by another intervention study that did not support that soy consumption can benefit men with prostate cancer, while not excluding the possibility of soy’s effect in preventing prostate cancer (39).
Mechanisms of soy’s cancer prevention effects are attributable to the isoflavones. Previous studies suggested that multiple mechanisms of action may be responsible. Isoflavones have been shown to affect the cell cycle, apoptosis, differentiation, proliferation, growth, along with effects on cell signaling (38,40,41). Isoflavones also exhibited antioxidant capacity. They are well-known scavengers for reactive oxygen species, but recent research is suggestive of additional antioxidant activity beyond direct scavenging of radicals. Genistein, in particular, has been shown to activate transcription factors such as estrogen receptor and stimulate gene expression in breast cancer cells (38). By contrast, genistein inhibited androgen responsive gene expression (42). These results indicating that genistein may have broad ability to impact overall cellular homeostasis through a variety of mechanisms/pathways.
Protection against other chronic diseases
Aside from the diseases mentioned above, soy consumption has also been linked to its effects in other chronic diseases, though not as extensively studied. Diabetes, cognitive function, and immune function are among the list of conditions that can potentially benefit from consumption of soy or soy components (25,43-45). As with the diseases discussed above, it remains unclear whether it is isoflavones, proteins, fiber, or some other component causing the supposed beneficial effects. The complexity of soy components makes it very hard to dissect out the exact active component(s) or compound(s) responsible for any particular bioactivity. Compounds may counteract with each other, or show distinct activities resulting from different concentration or ratio. Thus, the study of each individual component is proven to be necessary and important to gather scientific evidence and knowledge to support soy’s health-promoting effects.
Bioactive components of soy
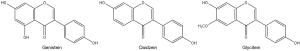
Isoflavones, tocopherols, carotenoids, and phytosterols are the main bioactive components reported in soy (1). Isoflavones are a class of polyphenolics found almost exclusively in legumes, and most prominently in soy. Isoflavones are structurally similar to that of estradiol, which binds weakly to estrogen receptors, eliciting weak estrogenic responses, and earning the title of ‘phytoestrogen’ (45). Genistein, daidzein, and glycitein are the predominating isoflavones in soy (Figure 1) (1). Biological activities of soy isoflavones include prevention against atherosclerosis and other CVD, obesity, osteoporosis, and cancer (24,46-50). Due to its estrogenic effect, genistein has been shown to alter the expression of genes involved in estrogen-mediated pathways and induce a proliferative response in breast cancer cells at 1–5 µM, while higher concentration (25 µM) of genistein can induce apoptosis and inhibition of proliferation (51). It was also reported that genistein and daidzein affect androgen responsive pathways in prostate cancer cells (52). These findings indicated that the biological activities of soy isoflavones are dose-dependent and involve multiple pathways. Recent research and clinical studies have provided a more comprehensive understanding of the effects and mechanism of actions of the soy isoflavones, however, the exact health outcomes remain elusive and warrant further studies.
Tocopherols are members of the Vitamin E family and can act as antioxidants by scavenging peroxyl radicals (1,53). Carotenoids also possess antioxidant activity, as their highly conjugated structures are extremely vulnerable to oxidation in the presence of light, heat, and oxygen (54). Sterols found in soybean oils are mostly in the non-esterified form. Plant sterols have a structural backbone very similar to that of human cholesterol. The esterified sterols have been shown to reduce serum cholesterol levels when consumed in sufficient quantities (55).
Other soy phytochemicals have not been studied as extensively. Recently, a novel group of soy phytoalexin, the glyceollins, was gathering great interest for their potential biological activities. The chemical and biological properties of glyceollins are reviewed in the following sections.
A novel group of soy phytochemicals: glyceollins
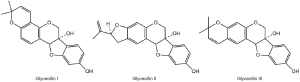
Glyceollins are one of the major groups of phytoalexins in soy. It was first reported in elicited soybean by Zahringer et al. in 1977 (56). A number of studies have been conducted to elucidate the biosynthesis of glyceollins in soybean. Banks and Dewick [1983] pointed out that phenylalanine, daidzein, 7,2’,4’-trihydroxyisoflavone, 3,9-dihydroxypterocarpan and glycinol are precursors for biosynthesis of glyceollin I, II and III (57). In the following decades, plant scientists had shown interest in glyceollins mainly for its antifungal and antibacterial effects in soybean (58-60), and a number of fungi were identified to be effective elicitors, e.g., Rhizopus oryzae (61), Mucor ramosissimus (62), Diaporthe Meridionalis (63), Aspergillus sojae (64). It was determined that glyceollins are in a relatively low amount in unstressed soy (1 to 9 g/g fresh weight of soy, depending on the different parts of soy). While, upon induction, glyceollins concentration can go up to 43 to 955 g/g fresh weight of soy, depending on the different elicitors and parts of soy (65-67). Besides effort in isolating glyceollins from elicited soy, chemical synthesis was also studied. However, due to the time-consuming and multiple steps needed in contiguous ring systems preparation, as well as the maintenance of rigorous stereo-control, only glyceollin I have been synthesized to date with limited preparation capacity (68-71).
Biological activities of soy phytochemical glyceollins
Phytoalexins are inducible chemicals involved in plants’ self-defense system (72), which have low molecular weight, possess anti-microbial activities, and are biosynthesized de novo in response to stress, including microbial attack, heavy metal salts, or UV radiation (73-75). Aside from the anti-microbial activity, some phytoalexins are also indicated to exhibit chronic disease prevention and health-promoting effects in humans (76).
Glyceollin I, II, and III (Figure 2) are the most common isomers isolated from soybean (57). A number of biological activities were reported by previous studies, including antiproliferation (77-79), antiestrogenic (80), antibacterial (81), antinematode (82,83), antifungal activities (84,85), insulinotropic (86) and attenuation of vascular contraction activity in the rat (87).
Insulinotropic effect
Glyceollins’ role in improving glucose homeostasis was reported by Park and colleagues (86,88) and they concluded that glyceollins act through regulating glucose utilization in adipocytes and modulating cell function and survival. It was shown that glyceollins could improve insulin-stimulated glucose uptake and decrease triacylglycerol accumulation in 3T3-L1 mouse adipocytes. It was shown that 5 µM glyceollins increased basal glucose uptake by 150%. Additionally, co-incubation of glyceollins and insulin further stimulated maximal glucose uptake above basal levels than that of either stimulus alone. Mechanistically, glucose transporter GLUT4 mRNA and protein expression significantly increased upon exposure of 5 µM glyceollins for 3 h in 3T3-L1 adipocytes (89). In addition, glyceollins slightly improved glucose-stimulated insulin secretion in Min6 pancreatic cells, and they potentiated insulinotropic actions in dysfunction cell. This was associated with decreased cell apoptosis because of the attenuation of endoplasmic reticulum stress. Glyceollins also potentiated GLP-1 secretion to enhance insulinotropic actions in enteroendocrine cells (86). Glyceollins treatment reduced blood glucose levels in diabetic mice and prediabetic rats in oral glucose tolerance testing. The improvement was associated with increased serum insulin levels, hepatic glycogen accumulation, and decreased triglyceride storage. It was proposed that glyceollins improved glucose homeostasis partly by enhancing hepatic insulin sensitivity in type 2 diabetic mice (88,89).
Antiestrogenic and antiproliferative effect
Both estrogenic and antiestrogenic activities have been identified in soy phytochemicals. Daidzein, the precursor of glyceollins, is known to be weakly estrogenic, while, glyceollins have been shown to be antiestrogenic (80,90). Glyceollins exert greater antagonism toward ER than ER in transiently transfected HEK 293 human embryonic kidney cells. It was also observed that glyceollins’ antiestrogenic effect on ER signaling could lead to a marked suppression of 17-estradiol-induced proliferation in MCF-7 cells (80). Among the three glyceollin isomers, it was shown that glyceollin I is the most potent antiestrogenic agent. Glyceollin I could effectively inhibit ERE transcription and endogenous gene expression in MCF-7 cells (78).
The antiestrogenic effect was further tested by Zimmermann et al. in athymic mice model and resulted in a 53.4% and 73.1% suppression of MCF-7 and BG-1 xenograft tumor growth, respectively (91). Trefoil factor 1 and progesterone receptor were reported to be affected by glyceollins treatment, and responsible for their breast cancer protective effect (92).
Furthermore, glyceollins were also noticed for their effect in suppressing tumorigenesis in triple-negative breast carcinoma MDA-MB-231 (ER-, PgR- and Her2/neu-) cells. Modest suppression of MDA-MB-231 and MDA-MB-468 cell tumor growth in vivo was observed upon glyceollins treatment, and a distinct change in microRNA expression profiles and proteomes in MDA-MB-231 was identified to be responsible for glyceollins’ effect. This study indicated that, aside from antiestrogenic effect, glyceollins could also exert antitumor activity in triple-negative breast carcinoma cell systems via alteration of microRNA and proteomic expression profiles (93).
Glyceollins’ antiproliferative effect was also studied in the LNCaP human prostate cancer cell. It was shown that glyceollins exerted the growth inhibitory effects through inhibition of G1/S progression and correlated with an up-regulation of CDKN1A and CDKN1B mRNA and protein levels. Furthermore, glyceollins inhibited LNCaP cell growth and cell cycle through a 17--estradiol-mediated event instead of an androgen-mediated event. In addition, glyceollin treatments led to down-regulated mRNA levels for androgen-responsive genes (94).
Antioxidation
Soy extract is known for its antioxidant capacity, and a number of phytochemicals have been identified, among them genistein and daidzein were the focus of previous studies (2,95,96). Structurally, glyceollins (Figure 2) are similar to genistein and daidzein (Figure 1). Glyceollins have also been reported for their antioxidant activity. Kim et al. reported that glyceollins possess a potent reducing ability, and can inhibit lipid peroxidation, scavenge radicals including singlet oxygen, superoxide anion, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), and 2,2-diphenyl-1-picrylhydrazyl (DPPH). In vitro model also indicated glyceollins significantly suppress H2O2-induced ROS production in hepa1c1c7 mouse hepatoma cells (64).
Additionally, glyceollins were shown to induce NADPH:quinone reductase in a dose-dependent manner in both Hepa1c1c7 mouse hepatoma and BPRc1 cells. Glyceollins also increased the expression of HO1, -GCL, and GR by promoting nuclear translocation of the Nrf2. Furthermore, glyceollins could upregulate phosphorylation of Akt and antioxidant response element-mediated reporter gene expression, which indicated that glyceollins may induce Nrf2-mediated phase 2 enzyme genes through activation of the PI3K signaling pathway (97).
Anti-inflammation
Anti-inflammatory effect of glyceollins was examined in RAW264.7 mouse macrophage cells. Glyceollins (0.3–3 g/mL) was able to inhibit NO production and iNOS, IL-6 and COX-2 gene expression induced by LPS in RAW264.7 mouse macrophage cells. Mechanistically, glyceollins were shown to suppress the LPS-induced phosphorylation of NF-B p65 and regulate NF-B activity (98).
Cholesterol-lowering effect
Hypercholesterolemia, the increased amounts of cholesterol in the blood, is a major factor contributing to the onset of cardiovascular disease. In a 2013 study, the cholesterol-lowering effect of glyceollins was investigated in a hamster model (99). Fed with a high fat diet containing 250 mg glyceollins per kg diet, significant reductions in plasma VLDL, hepatic cholesterol esters and total lipid content were achieved compared to the control group fed a high fat diet. Consistent with changes in circulating cholesterol, glyceollins supplementation also altered expression of the genes related to cholesterol metabolism in the liver.
Summary
Decades of research and investigation have revealed many aspects of soy and soy foods’ health-promoting effects, including protection against cardiovascular diseases, obesity-related metabolic syndrome, several types of cancers, and other chronic diseases. Soy phytochemicals are an important group of soy components and have been shown to contribute to soy’s beneficial effects. Glyceollins, as a novel soy phytochemical, have shown promising biological effects, such as insulinotropic, antiestrogenic, antiproliferative, antioxidative, anti-inflammatory and cholesterol-lowering effects. The understanding of soy phytochemicals’ mechanism of action can help and guide the use of soy as a health-promoting food. In addition to the study of biological effects of soy and its phytochemicals, to fully realize the potential of soy, two key factors will also have to be taken into account. Firstly, to target one condition or a specific pathway without taking into consideration of the impact of such effect(s) on other conditions or pathways may not result in the overall improvement of health. The basic research can help accumulate the data and knowledge in an ever-increasing list of effects and mechanism of actions, while the actual health promotion will have to result from a comprehensive understanding and a balance of these effects. Secondly, the era of treating the population as an average is getting to an end. The individual health condition, genetic background, external factors (environmental factors, residing microbiome, and lifestyles, etc.) shall all be accounted for to render the expected benefits effective. Such holistic approach is being made possible by the advance of the life science and medical research and shall guide the future of human health promotion.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.10.04). Dr. Li serves as an unpaid section editor of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu KS. Soybeans: Chemistry, Technology and Utilization. New York, NY, USA: Springer, 1997.
- Tripathi AK, Misra AK. Soybean - a consummate functional food: A review. Journal of Food Science and Technology Mysore 2005;42:111-9.
- FAOSTAT [database on the Internet] 2013. Available online: http://www.fao.org/faostat/en/#data/QC
- Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed Regist 1999;64:57700-33. [PubMed]
- United Soybean Board. Consumer Attitudes About Nutrition, Health and Soyfoods. 21st Annual Survey 2014.
- Wansink B, van Ittersum K, Painter JE. How descriptive food names bias sensory perceptions in restaurants. Food Qual Prefer 2005;16:393-400. [Crossref]
- Schyver T, Smith C. Reported attitudes and beliefs toward soy food consumption of soy consumers versus nonconsumers in natural foods or mainstream grocery stores. J Nutr Educ Behav 2005;37:292-9. [Crossref] [PubMed]
- Wansink B, Chan N. Relation of soy consumption to nutritional knowledge. Journal of Medicinal Food 2001;4:145-50. [Crossref] [PubMed]
- Sacks FM, Lichtenstein A, Van Horn L, et al. Soy protein, isoflavones, and cardiovascular health - An American heart association science advisory for professionals from the nutrition committee. Circulation 2006;113:1034-44. [Crossref] [PubMed]
- Sirtori CR, Galli C, Anderson JW, et al. Functional foods for dyslipidaemia and cardiovascular risk prevention. Nutr Res Rev 2009;22:244-61. [Crossref] [PubMed]
- Clerici C, Setchell K, Pirro M, et al. Isoflavones in food with minimal soy protein reduce serum cholesterol and improve important markers of cardiovascular risk. J Nutr 2004;134:1268S-1269S.
- Xiao CW. Health effects of soy protein and isoflavones in humans. J Nutr 2008;138:1244S-1249S. [PubMed]
- Ghosh D, Scheepens A. Vascular action of polyphenols. Mol Nutr Food Res 2009;53:322-31. [Crossref] [PubMed]
- Ghosh D. Potential role of polyphenol-fortified foods and beverages on vascular health. Agro Food Industry Hi Tech 2009;20:25-6.
- Anderson JW, Johnstone BM, Cooknewell ME. Metaanalysis of the effects of soy protein-intake on serum-lipids. N Engl J Med 1995;333:276-82. [Crossref] [PubMed]
- Takahashi R, Ohmori R, Kiyose C, et al. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J Agric Food Chem 2005;53:4578-82. [Crossref] [PubMed]
- Astadi IR, Astuti M, Santoso U, et al. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low density lipoprotein (LDL). Food Chem 2009;112:659-63. [Crossref]
- Rho SJ, Park S, Ahn CW, et al. Dietetic and hypocholesterolaemic action of black soy peptide in dietary obese rats. J Sci Food Agric 2007;87:908-13. [Crossref]
- Rho SJ, Lee JS, Il Chung Y, et al. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochemistry 2009;44:490-3. [Crossref]
- Kong JM, Chia LS, Goh NK, et al. Analysis and biological activities of anthocyanins. Phytochemistry 2003;64:923-33. [Crossref] [PubMed]
- de Pascual-Teresa S, Moreno DA, Garcia-Viguera C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int J Mol Sci 2010;11:1679-703. [Crossref] [PubMed]
- Van Horn L, McCoin M, Kris-Etherton PM, et al. The evidence for dietary prevention and treatment of cardiovascular disease. J Am Diet Assoc 2008;108:287-331. [Crossref] [PubMed]
- Azadbakht L, Esmaillzadeh A. Soy and cardio-metabolic abnormalities: an update. J Res Med Sci 2008;13:88-96.
- Ørgaard A, Jensen L. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 2008;233:1066-80. [Crossref] [PubMed]
- Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 2002;76:1191-201. [PubMed]
- Oba S, Nagata C, Shimizu N, et al. Soy product consumption and the risk of colon cancer: A prospective study in Takayama, Japan. Nutr Cancer 2007;57:151-7. [Crossref] [PubMed]
- Nagata Y, Sonoda T, Mori M, et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr 2007;137:1974-9. [PubMed]
- Messina M. Resolving the soy-breast cancer controversy. J Am Diet Assoc 2006;106:363-4. [Crossref] [PubMed]
- Valachovicova T, Slivova V, Sliva D. Cellular and physiological effects of soy flavonoids. Mini Rev Med Chem 2004;4:881-7. [Crossref] [PubMed]
- Wu AH, Yu MC, Tseng CC, et al. Epidemiology of soy exposures and breast cancer risk. Br J Cancer 2008;98:9-14. [Crossref] [PubMed]
- Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst 2006;98:459-71. [Crossref] [PubMed]
- Magee PJ, Rowland I. Soy products in the management of breast cancer. Curr Opin Clin Nutr Metab Care 2012;15:586-91. [Crossref] [PubMed]
- Messina MJ, Wood CE. Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J 2008;7:17. [Crossref] [PubMed]
- Messina M. Conclusion that isoflavones exert estrogenic effects on breast tissue and may raise breast cancer risk unfounded. Mol Nutr Food Res 2008;52:299-300. [Crossref] [PubMed]
- Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr 2009;89:1673S-1679S. [Crossref] [PubMed]
- Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev 2003;61:117-31. [Crossref] [PubMed]
- Ahmad IU, Forman JD, Sarkar F, et al. Reduction of adverse events by soy isoflavones in patients undergoing external beam radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:S318. [Crossref]
- Banerjee S, Li YW, Wang ZW, et al. Multi-targeted therapy of cancer by genistein. Cancer Lett 2008;269:226-42. [Crossref] [PubMed]
- Bosland MC, Kato I, Melamed J, et al. Chemoprevention trials in men with prostate-specific antigen failure or at high risk for recurrence after radical prostatectomy: Application to efficacy assessment of soy protein. Urology 2001;57:202-4. [Crossref] [PubMed]
- Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int 2006;89:1121-34. [PubMed]
- Zhou JR, Gugger ET, Tanaka T, et al. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr 1999;129:1628-35. [PubMed]
- Lazarevic B, Karlsen SJ, Saatcioglu F. Genistein differentially modulates androgen-responsive gene expression and activates JNK in LNCaP cells. Oncol Rep 2008;19:1231-5. [PubMed]
- Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem 2005;16:641-9. [Crossref] [PubMed]
- Ryan-Borchers TA, Park JS, Chew BP, et al. Soy isoflavones modulate immune function in healthy postmenopausal women. Am J Clin Nutr 2006;83:1118-25. [PubMed]
- Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Mol Cell Endocrinol 2009;304:30-42. [Crossref] [PubMed]
- Sarkar FH, Li YW. Soy isoflavones and cancer prevention. Cancer Invest 2003;21:744-57. [Crossref] [PubMed]
- Clair RS, Anthony M. Soy, isoflavones and atherosclerosis. Handb Exp Pharmacol 2005;301-23. [Crossref] [PubMed]
- Weaver CM, Cheong JMK. Soy isoflavones and bone health: The relationship is still unclear. J Nutr 2005;135:1243-7. [PubMed]
- Taku K, Melby MK, Nishi N, et al. Soy isoflavones for osteoporosis: An evidence-based approach. Maturitas 2011;70:333-8. [Crossref] [PubMed]
- Gil-Izquierdo A, Penalvo JL, Gil JI, et al. Soy Isoflavones and Cardiovascular Disease Epidemiological, Clinical and - Omics Perspectives. Curr Pharm Biotechnol 2012;13:624-31. [Crossref] [PubMed]
- Lavigne JA, Takahashi Y, Chandramouli GVR, et al. Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res Treat 2008;110:85-98. [Crossref] [PubMed]
- Takahashi Y, Lavigne JA, Hursting SD, et al. Molecular signatures of soy-derived phytochemicals in androgen-responsive prostate cancer cells: A comparison study using DNA microarray. Mol Carcinog 2006;45:943-56. [Crossref] [PubMed]
- Britz SJ, Kremer DF, Kenworthy WJ. Tocopherols in soybean seeds: Genetic variation and environmental effects in field-grown crops. Journal of the American Oil Chemists Society 2008;85:931-6. [Crossref]
- Lee JD, Shannon JG, So YS, et al. Environmental effects on lutein content and relationship of lutein and other seed components in soybean. Plant Breeding 2009;128:97-100. [Crossref]
- Phillips KM, Ruggio DM, Toivo JI, et al. Free and esterified sterol composition of edible oils and fats. J Food Compost Anal 2002;15:123-42. [Crossref]
- Zahringer U, Ebel J, Kreuzaler F, et al. Biosynthesis of elicitor-induced phytoalexin, glyceollin in soybean (glycine max). Hoppe-Seylers Zeitschrift Fur Physiologische Chemie 1977;358:1303-4.
- Banks SW, Dewick PM. Biosynthesis of glyceollin-i, glyceollin-ii and glycellin-iii in soybean. Phytochemistry 1983;22:2729-33. [Crossref]
- Olah AF, Schmitthenner AF, Walker AK. The role of glyceollin in soybean root tolerance to phytophthora root-rot. Phytopathology 1982;72:967.
- Wyss P, Boller T, Wiemken A. Phytoalexin response is elicited by a pathogen (rhizoctonia-solani) but not by a mycorrhizal fungus (glomus-mosseae) in soybean roots. Experientia 1991;47:395-9. [Crossref]
- Parniske M, Fischer HM, Hennecke H, et al. Accumulation of the phytoalexin glyceollin i in soybean nodules infected by a bradyrhizobium-japonicum-nifa mutant. Zeitschrift für Naturforschung C 1991;46:318-20.
- Simons R, Vincken JP, Roidos N, et al. Increasing soy isoflavonoid content and diversity by simultaneous malting and challenging by a fungus to modulate estrogenicity. J Agric Food Chem 2011;59:6748-58. [Crossref] [PubMed]
- Garcez WS, Martins D, Garcez FR, et al. Effect of spores of saprophytic fungi on phytoalexin accumulation in seeds of frog-eye leaf spot and stem canker-resistant and -susceptible soybean (Glycine max L.) cultivars. J Agric Food Chem 2000;48:3662-5. [Crossref] [PubMed]
- Modolo LV, Cunha FQ, Braga MR, et al. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp meridionalis elicitor. Plant Physiol 2002;130:1288-97. [Crossref] [PubMed]
- Kim HJ, Suh HJ, Kim JH, et al. Antioxidant Activity of Glyceollins Derived from Soybean Elicited with Aspergillus sojae. J Agric Food Chem 2010;58:11633-8. [Crossref] [PubMed]
- Degousee N, Triantaphylides C, Montillet JL. Involvement of oxidative processes in the signaling mechanisms leading to the activation of glyceollin synthesis in soybean (glycine-max). Plant Physiol 1994;104:945-52. [Crossref] [PubMed]
- Kraus C, Spiteller G, Mithöfer A, et al. Quantification of glyceollins in non-elicited seedlings of glycine-max by gas chromatography mass spectrometry. Phytochemistry 1995;40:739-43. [Crossref]
- Boué SM, Carter CH, Ehrlich KC, et al. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J Agric Food Chem 2000;48:2167-72. [Crossref] [PubMed]
- Khupse RS, Erhardt PW. Total Syntheses of Racemic, Natural (-) and Unnatural (+) Glyceollin I. Org Lett 2008;10:5007-10. [Crossref] [PubMed]
- Luniwal A, Khupse RS, Reese M, et al. Total Syntheses of Racemic and Natural Glycinol. J Nat Prod 2009;72:2072-5. [Crossref] [PubMed]
- Khupse RS, Sarver JG, Trendel JA, et al. Biomimetic Syntheses and Antiproliferative Activities of Racemic, Natural (-), and Unnnatural (+) Glyceollin I. J Med Chem 2011;54:3506-23. [Crossref] [PubMed]
- Luniwal A, Khupse R, Reese M, et al. Multigram Synthesis of Glyceollin I. Org Process Res Dev 2011;15:1149-62. [Crossref]
- Jeandet P, Douillt-Breuil AC, Bessis R, et al. Phytoalexins from the vitaceae: biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J Agric Food Chem 2002;50:2731-41. [Crossref] [PubMed]
- Chamberl DW, Paxton JD. Protection of soybean plants by phytoalexin. Phytopathology 1968;58:1349-50.
- Paxton JD. Inducer of soybean phytoalexin. Phytopathology 1971;61:1025.
- Murch RS, Paxton JD. Environmental stress and phytoalexin accumulation in soybean. Bulletin De La Societe Botanique De France-Actualites Botaniques 1980;127:151-3. [Crossref]
- Boue SM, Cleveland TE, Carter-Wientjes C, et al. Phytoalexin-Enriched Functional Foods. J Agric Food Chem 2009;57:2614-22. [Crossref] [PubMed]
- Lee MR, Kim JY, Chun J, et al. Induction of Glyceollins by Fungal Infection in Varieties of Korean Soybean. J Microbiol Biotechnol 2010;20:1226-9. [Crossref] [PubMed]
- Payton-Stewart F, Khupse RS, Boue SM, et al. Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Steroids 2010;75:870-8. [Crossref] [PubMed]
- Salvo VA, Boue SM, Fonseca JP, et al. Antiestrogenic glyceollins suppress human breast and ovarian carcinoma tumorigenesis. Clin Cancer Res 2006;12:7159-64. [Crossref] [PubMed]
- Burow ME, Boue SM, Collins-Burow BM, et al. Phytochemical glyceollins, isolated from soy, mediate antihormonal effects through estrogen receptor alpha and beta. J Clin Endocrinol Metab 2001;86:1750-8. [PubMed]
- Weinstein LI, Albersheim P. Host-Pathogen Interactions: XXIII. The mechanism of the anti-bacterial action of glycinol, a pterocarpan phytoalexin synthesized by soybeans. Plant Physiol 1983;72:557-63. [Crossref] [PubMed]
- Veech JA. Phytoalexins and their role in the resistance of plants to nematodes. J Nematol 1982;14:2-9. [PubMed]
- Huang JS, Barker KR. Glyceollin-i in soybean-cyst nematode interactions - spatial and temporal distribution in roots of resistant and susceptible soybeans. Plant Physiol 1991;96:1302-7. [Crossref] [PubMed]
- Lozovaya VV, Lygin AV, Zernova OV, et al. Isoflavonoid accumulation in soybean hairy roots upon treatment with Fusarium solani. Plant Physiol Biochem 2004;42:671-9. [Crossref] [PubMed]
- Lee YS, Kim HK, Lee KJ, et al. Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep 2010;43:461-7. [Crossref] [PubMed]
- Park S, Ahn IS, Kim JH, et al. Glyceollins, One of the Phytoalexins Derived from Soybeans under Fungal Stress, Enhance Insulin Sensitivity and Exert Insulinotropic Actions. J Agric Food Chem 2010;58:1551-7. [Crossref] [PubMed]
- Song MJ, Baek I, Jeon SB, et al. Effects of glyceollin I on vascular contraction in rat aorta. Naunyn Schmiedebergs Arch Pharmacol 2010;381:517-28. [Crossref] [PubMed]
- Park S, Kim DS, Kim JH, et al. Glyceollin-containing fermented soybeans improve glucose homeostasis in diabetic mice. Nutrition 2012;28:204-11. [Crossref] [PubMed]
- Boué SM, Isakova IA, Burow ME, et al. Glyceollins, Soy Isoflavone Phytoalexins, Improve Oral Glucose Disposal by Stimulating Glucose Uptake. J Agric Food Chem 2012;60:6376-82. [Crossref] [PubMed]
- Jiang Q, Payton-Stewart F, Elliott S, et al. Effects of 7-O Substitutions on Estrogenic and Anti-Estrogenic Activities of Daidzein Analogues in MCF-7 Breast Cancer Cells. J Med Chem 2010;53:6153-63. [Crossref] [PubMed]
- Zimmermann MC, Tilghman SL, Boue SM, et al. Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy. J Pharmacol Exp Ther 2010;332:35-45. [Crossref] [PubMed]
- Wood CE, Clarkson TB, Appt SE, et al. Effects of soybean glyceollins and estradiol in postmenopausal female monkeys. Nutr Cancer 2006;56:74-81. [Crossref] [PubMed]
- Rhodes LV, Tilghman SL, Boue SM, et al. Glyceollins as novel targeted therapeutic for the treatment of triple-negative breast cancer. Oncol Lett 2012;3:163-71. [PubMed]
- Payton-Stewart F, Schoene NW, Kim YS, et al. Molecular Effects of Soy Phytoalexin Glyceollins in Human Prostate Cancer Cells LNCaP. Mol Carcinog 2009;48:862-71. [Crossref] [PubMed]
- Slavin M, Cheng ZH, Luther M, et al. Antioxidant properties and phenolic, isoflavone, tocopherol and carotenoid composition of Maryland-grown soybean lines with altered fatty acid profiles. Food Chem 2009;114:20-7. [Crossref]
- Slavin M, Kenworthy W, Yu L. Antioxidant Properties, Phytochemical Composition, and Antiproliferative Activity of Maryland-Grown Soybeans with Colored Seed Coats. J Agric Food Chem 2009;57:11174-85. [Crossref] [PubMed]
- Kim HJ, di Luccio E, Kong AN, et al. Nrf2-mediated induction of phase 2 detoxifying enzymes by glyceollins derived from soybean exposed to Aspergillus sojae. Biotechnol J 2011;6:525-36. [Crossref] [PubMed]
- Kim HJ, Sung MK, Kim JS. Anti-inflammatory effects of glyceollins derived from soybean by elicitation with Aspergillus sojae. Inflamm Res 2011;60:909-17. [Crossref] [PubMed]
- Huang H, Xie Z, Boue SM, et al. Cholesterol-Lowering Activity of Soy-Derived Glyceollins in the Golden Syrian Hamster Model. J Agric Food Chem 2013;61:5772-82. [Crossref] [PubMed]
Cite this article as: Wu H, Zhang Z, Huang H, Li Z. Health benefits of soy and soy phytochemicals. AME Med J 2017;2:162.