
Noninvasive diagnosis of nonalcoholic fatty liver disease, is the more expensive the better?
Introduction
Nonalcoholic fatty liver disease (NAFLD) may be defined as a sexual dimorphic disease (1) featuring excess intrahepatic ectopic triglyceride deposition in patients who are free of competing etiologies of liver disease (2). NAFLD has a close, mutual and bi-directional relationship with metabolic syndrome (MetS), of which it may be either a cause (3) or an effect (4).
NAFLD, globally the most frequent liver disease, and projected to further increase (5), is associated with a wide spectrum of hepatic disorders ranging from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma (6). Moreover, frequent co-morbidities of NAFLD, which characterize its natural course in the individual patient, include specific cardio-renal-metabolic conditions and increased hepatic/extrahepatic cancer risk (7).
The diagnosis of NAFLD
The current reference standard in diagnosing NAFLD is liver biopsy, a relatively invasive procedure whose accuracy and safety has been challenged based on potential complications, sampling errors, sub-optimal intra- and inter-observer agreement (8).
The three elementary liver changes based on which the various histological scoring systems establish the diagnosis of NAFLD include: steatosis, necro-inflammatory changes, and fibrosis (1,8). Can these three elementary histological features be diagnosed other than through liver biopsy? A variety of "wet" (chemical) and "dry" (physical) tools have been proposed to this end (8). However, the most eagerly awaited by clinicians are those evaluating liver fibrosis given that it is this elementary histological change which dictates the prognosis of hepatic and extrahepatic course of disease (7,8).
Imaging techniques, such as the cheaper and more widely available ultrasonography-based techniques, and the more resource-consuming and poorly diffuse magnetic resonance imaging (MRI)-based techniques, may detect steatosis and fibrosis non-invasively.
As regards ultrasonography-based techniques, vibration controlled transient elastography (VCTE; FibroScan®) allows assessment of hepatic tissue stiffness. VCTE accurately predicts, in particular, the more advanced stages of fibrosis thus reducing the number of candidates to undergo liver biopsy (8,9). Through the controlled attenuation parameter (CAP), VCTE (FibroScan®) will also simultaneously assess steatosis (8,9). The clinical relevance of assessing steatosis is more uncertain than that of fibrosis though some authors tend to believe steatosis to be correlated with an increased cardiovascular risk (10). Ultrasonography-based techniques may be applied with difficulty in the morbid obese subject. The availability of XL probes partly overcomes this shortcoming.
Compared to ultrasonography-based imaging techniques, those based on MRI, such as magnetic resonance elastography (MRE) and proton density fat fraction (MRI-PDFF), tend to be more accurate and they are able to accurately diagnose fibrosis and steatosis also in NAFLD patients with morbid obesity (11-17), though they are more expensive and far less largely available globally (18).
The paper by Park and colleagues
Based on previous Asian studies, Park et al. hypothesized that MRE was superior to VCTE in diagnosing early fibrosis, and MRI-PDFF superior to CAP for diagnosing steatosis also in a Western NAFLD population (19). In order to demonstrate their working hypothesis, these authors conducted a prospective, cross-sectional study of 104 American adult patients with suspected NAFLD who underwent contemporaneous MRI and VCTE, including the use of XL probe when indicated, with a liver biopsy assessment to compare the accuracy of VCTE versus MRE for diagnosing fibrosis, and CAP versus MRI-PDFF for diagnosing steatosis in NAFLD patients (19).
Data have shown that MRI-based MRE and MRI-PDFF are significantly more accurate than ultrasonography-based VCTE and CAP, respectively, for diagnosing any fibrosis (stage 1–4 vs. 0) and all dichotomized grades of hepatic steatosis in an American cohort of patients with biopsy-proven NAFLD (19). However, no significant difference was found between MRE and VCTE for diagnosing other dichotomized stages of fibrosis (19).
Authors conclude that MRI-based techniques may be preferable to ultrasonography-based techniques for accurate non-invasive assessment of NAFLD. However, the cost effectiveness of utilizing MRE/MRI-PDFF versus VCTE/CAP and/or biopsy should also be further evaluated to develop optimal diagnostic strategies for diagnosing NAFLD-associated fibrosis and steatosis (19).
Conclusions
European clinical guidelines on the management of NAFLD were issued in 2016 by the three scientific societies of liver disease, diabetes and obesity (20). As one of their most distinctive features, such guidelines raised considerable concern about the difficulties expected in conducting universal screening campaigns and appropriate surveillance strategies and follow-up. This is due to the innumerable population of individuals at risk of NAFLD which greatly outnumbers those resources that National healthcare systems can afford to invest (20).
We believe that such concerns cannot be underrated and should always be kept in mind. Moreover, local availability and expertise are likely to remain major determinants of the diagnostic strategy which is implementable. It should not be forgotten that there are countries in which patients to be submitted to standard liver ultrasonography are fully scrutinized (21) and that the rich diagnostic potential of the cheap and widely available semi-quantitative ultrasonographic indices remain to be fully exploited (22).
Collectively, data commented in the present editorial may raise the expectation that, as regards NAFLD: in academic research conducted in developed countries the most accurate and expensive MRI-based imaging techniques will increasingly become the standard of care; in clinical practice ultrasonography-based techniques will probably retain their prominent role worldwide; in some developing countries access to ultrasound-based techniques will possibly remain precluded for most suspected NAFLD cases.
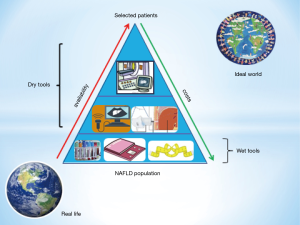
On this background, the challenge for the future will be to reduce the costs and to increase the availability of the most accurate and expensive diagnostic tools (Figure 1). Meanwhile, a more extensive exploitation of the potential of ultrasonographic technique (22) should be encouraged.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Han Deng (Gastroenterology and Hepatology, Yuebei People’s Hospital, Shaoguan, China).
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.11.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ballestri S, Nascimbeni F, Baldelli E, et al. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291-326. [Crossref] [PubMed]
- Petäjä EM, Yki-Järvinen H. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD-A Systematic Review. Int J Mol Sci 2016;17:633. [Crossref] [PubMed]
- Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016;31:936-44. [Crossref] [PubMed]
- Ballestri S, Nascimbeni F, Romagnoli D, et al. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol Res 2016;46:1074-87. [Crossref] [PubMed]
- Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2017; [Epub ahead of print]. [Crossref] [PubMed]
- Ballestri S, Nascimbeni F, Romagnoli D, et al. The role of nuclear receptors in the pathophysiology, natural course, and drug treatment of NAFLD in Humans. Adv Ther 2016;33:291-319. [Crossref] [PubMed]
- Italian Association for the Study of the Liver (AISF). AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig Liver Dis 2017;49:471-83. [Crossref] [PubMed]
- Lonardo A, Nascimbeni F, Maurantonio M, et al. Nonalcoholic fatty liver disease: Evolving paradigms. World J Gastroenterol 2017;23:6571-92. [Crossref] [PubMed]
- Ballestri S, Romagnoli D, Nascimbeni F, et al. Role of ultrasound in the diagnosis and treatment of nonalcoholic fatty liver disease and its complications. Expert Rev Gastroenterol Hepatol 2015;9:603-27. [Crossref] [PubMed]
- Adams LA. NAFLD. Accurate quantification of hepatic fat--is it important? Nat Rev Gastroenterol Hepatol 2015;12:126-7. [Crossref] [PubMed]
- Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. [Crossref] [PubMed]
- Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology 2008;135:299-302. [Crossref] [PubMed]
- Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60:1920-8. [Crossref] [PubMed]
- Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther 2015;41:1271-80. [Crossref] [PubMed]
- Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology 2016;63:453-61. [Crossref] [PubMed]
- Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012;36:22-9. [Crossref] [PubMed]
- Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015;274:416-25. [Crossref] [PubMed]
- Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol 2016;65:1006-16. [Crossref] [PubMed]
- Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017;152:598-607.e2. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Saokaew S, Kanchanasuwan S, Apisarnthanarak P, et al. Clinical risk scoring for predicting non-alcoholic fatty liver disease in metabolic syndrome patients (NAFLD-MS score). Liver Int. 2017;37:1535-43. [Crossref] [PubMed]
- Ballestri S, Nascimbeni F, Baldelli E, et al. Ultrasonographic fatty liver indicator detects mild steatosis and correlates with metabolic/histological parameters in various liver diseases. Metabolism 2017;72:57-65. [Crossref] [PubMed]
Cite this article as: Lonardo A, Marrazzo A, Baldelli E, Nascimbeni F. Noninvasive diagnosis of nonalcoholic fatty liver disease, is the more expensive the better? AME Med J 2017;2:171.

