
Percutaneous patent foramen ovale closure for cryptogenic ischemic stroke: is it time for new guidelines?
Patent foramen ovale (PFO) mediated right-to-left shunting has been associated with a variety of medical syndromes including cryptogenic stroke, migraine headache, platypnea-orthodeoxia, sleep apnea and decompression sickness (1-7). Observational studies suggest that PFO occurs in 20–25% of the adult population, but up to 50% of patients with cryptogenic stroke have a PFO (8). While the etiology of PFO mediated stroke is attributed to paradoxical embolism, there is no increased benefit of anticoagulation with warfarin or newer oral anticoagulants for secondary prevention of cryptogenic stroke, in the absence of atrial fibrillation (9,10). Given the lack of a superior antithrombotic medication, non-randomized studies were performed to assess the efficacy of percutaneous PFO closure; these observational studies suggested that device closure reduces the rate of recurrent stroke compared with medical therapy in patients with cryptogenic ischemic stroke (1,8). Subsequently, five randomized controlled trials were completed in an effort to confirm these findings.
Contradicting previous observational studies, three earlier randomized trials failed to demonstrate superiority of percutaneous PFO closure over medical therapy for secondary prevention of stroke (11-13). Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale (CLOSURE I) trial was the first of these trials, which compared PFO closure with the STARFlex device (NMT Medical, Boston, Massachusetts, USA) to medical therapy in patients with an initial cryptogenic ischemic cerebrovascular event (11). The trial failed to demonstrate greater efficacy with closure for secondary stroke prevention, and was criticized for using a device that was associated with a high incidence of thrombosis, atrial fibrillation, and residual right-to-left shunting (14,15). The Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism (PC) trial randomized patients with cryptogenic embolism to closure with the Amplatzer PFO occluder (Abbott; Chicago, Illinois, USA) or medical therapy. While the Amplatzer device was found to be safe with no increased rate of serious adverse events, bleeding, atrial fibrillation or thrombosis, only a non-significant trend was seen favoring PFO closure (12). Failure of the PC trial to correlate with prior observational studies has been attributed to the trial being underpowered with risk of type II error. Additionally, the study included a cohort that was different than the observational studies (patients with transient ischemic attack and non-cerebral peripheral embolism) (15,16). While early results of the Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment (RESPECT) trial did not show greater efficacy with the Amplatzer device in an intention-to-treat analysis (13), the long-term follow-up data demonstrated a 45% relative risk reduction in recurrent stroke and a 62% relative risk reduction in recurrent cryptogenic stroke, at a median follow-up of 5.9 years (17). Efficacy of closure for secondary stroke prevention was even greater in the subset of patients with a large right-to-left shunt or atrial septal aneurysm. The rate of serious adverse events, major bleeding, thrombosis, and atrial fibrillation were all similar between patients who received the Amplatzer device compared with the medical therapy arm (P>0.05 for all). All of the earlier trials were limited by slow patient recruitment and lack of blinding, which may have potentiated the use of an off-label PFO occluding device in those randomized to the medical therapy arm.
A subsequent patient level meta-analysis of the early three trials further confirmed that percutaneous PFO closure has greater efficacy compared to medical therapy, for reducing the risk of recurrent stroke in patients with cryptogenic stroke (18). Based on the extended follow-up data from RESPECT and meta-analysis of these trials, the Food and Drug Administration of the United States subsequently approved the Amplatzer PFO occluder for use in patients with cryptogenic stroke presumed to be from paradoxical embolism by a cardiologist and neurologist (19).
More recently, the Patent Foramen Ovale Closure or Anticoagulants versus Antiplatelet Therapy to Prevent Stroke Recurrence (CLOSE) and the GORE® HELEX® Septal Occluder/GORE® CARDIOFORM Septal Occluder for PFO Closure in Stroke Patients (Gore REDUCE) trials were published (20,21). In the CLOSE trial, PFO closure was found to be more efficacious for secondary prevention of stroke compared to antiplatelet medical therapy (0% versus 6.0%, HR 0.03; 95% CI: 0–0.26; P<0.001) after a mean follow-up of 5.3±2.0 years. The Gore REDUCE trial corroborated these findings showing less recurrent stroke events in patients with a device (1.4% versus 5.4%, HR 0.23; 95% CI: 0.09–0.62; P=0.002) after a median follow-up of 3.2 years (Figure 1). Both trials reported no difference in major adverse events or bleeding between closure and medical therapy; however, patients with a device had over four-fold greater incidence of atrial fibrillation in both studies (P<0.05). We speculate that success of these new trials is primarily due to enrollment of patients who had ischemic stroke that was more truly cryptogenic and more likely a result of paradoxical embolism (22). The Gore REDUCE trial utilized extensive computed tomography and magnetic resonance cerebrovascular imaging to exclude stroke from large-artery atherosclerotic disease and lacunar infarcts. Additionally, the trial omitted patients with uncontrolled risk factors and most randomized patients underwent longer inpatient atrial fibrillation monitoring. The CLOSE trial strictly included patients with echocardiographic features predictive of greatest benefit with PFO closure; all included subjects had an atrial septal aneurysm or large shunt.
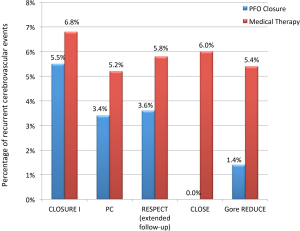
A recent meta-analysis of the five randomized trials further corroborated that percutaneous PFO closure lowers the risk of recurrent stroke compared with medical therapy in patients with cryptogenic stroke (2.0% versus 4.5%; RR 0.42, P=0.027) at a 2.9-year mean follow-up. The study also showed a four-fold increased risk of atrial fibrillation in patients with a device, with the risk being device dependent (Figure 2) (23). The majority of post-device atrial fibrillation episodes in the trials were a single event resolving either without intervention or with cardioversion.
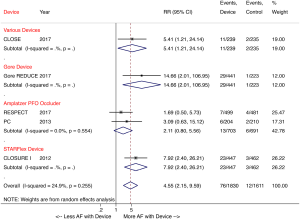
In summary, as demonstrated by the extended follow-up of the RESPECT trial, the CLOSE and Gore REDUCE trials, and meta-analyses of these trials, PFO occluding devices reduce the risk of recurrent stroke compared to medical therapy in patients with cryptogenic stroke. The new trials have allowed us to appreciate that rigorous exclusion criteria are vital, to identify patients who have stroke that is truly cryptogenic. While the clinical trials demonstrated that percutaneous PFO closure is safe, with no difference in major adverse events or bleeding between closure and medical therapy, deployment of these devices is known to irritate the atrial septum and predispose patients to post-implant atrial fibrillation. Given the high risk of post-closure atrial arrhythmias, patients should additionally undergo prolonged (≥30 days) atrial fibrillation monitoring to rule out paroxysmal atrial fibrillation as a cause of stroke, prior to recommending PFO closure (24,25). Guidelines must be updated to reflect the existing data, wherein percutaneous PFO closure should be recommended as first line therapy for all patients aged ≤60 years old with cryptogenic ischemic stroke. While those with an atrial septal aneurysm or large right-to-left shunt receive the greatest benefit from closure, current randomized data confirms that PFO closure should not be restricted to only these subset of patients.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Rui Liu (Department of Neurology, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China).
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.11.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agarwal S, Bajaj NS, Kumbhani DJ, et al. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv 2012;5:777-89. [Crossref] [PubMed]
- Khessali H, Mojadidi MK, Gevorgyan R, et al. The effect of patent foramen ovale closure on visual aura without headache or typical aura with migraine headache. JACC Cardiovasc Interv 2012;5:682-7. [Crossref] [PubMed]
- Mojadidi MK, Dave N, Gevorgyan R, et al. The Association of Patent Foramen Ovale and Migraine Headache. In: Amin Z, Tobis J, Sievert H, et al. editors. Patent Foramen Ovale. London: Springer, 2015:81-94.
- Mahmoud AN, Elgendy IY, Agarwal N, et al. Identification and Quantification of Patent Foramen Ovale-Mediated Shunts: Echocardiography and Transcranial Doppler. Interv Cardiol Clin 2017;6:495-504. [Crossref] [PubMed]
- Mojadidi MK, Gevorgyan R, Noureddin N, et al. The effect of patent foramen ovale closure in patients with platypnea-orthodeoxia syndrome. Catheter Cardiovasc Interv 2015;86:701-7. [Crossref] [PubMed]
- Mojadidi MK, Bokhoor PI, Gevorgyan R, et al. Sleep Apnea in Patients with and without a Right-to-Left Shunt. J Clin Sleep Med 2015;11:1299-304. [Crossref] [PubMed]
- Wilmshurst PT. The role of persistent foramen ovale and other shunts in decompression illness. Diving Hyperb Med 2015;45:98-104. [PubMed]
- Lechat P, Mas JL, Lascault G, et al. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988;318:1148-52. [Crossref] [PubMed]
- Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med 2001;345:1444-51. [Crossref] [PubMed]
- Bayer’s NAVIGATE ESUS study halted early as it indicated comparable efficacy between treatment arms. Available online: http://press.bayer.com/baynews/baynews.nsf/id/Bayers-NAVIGATE-ESUS-study-halted-early-as-it-indicated-comparable-efficacy-between-treatment-arms?OpenDocument&sessionID=1508870994 (accessed October 24, 2017).
- Buksińska Lisik M. Summary of the article: Furlan A J, Reisman M, Massaro J et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med, 2012;366:991-9. Kardiol Pol 2012;70:874. [PubMed]
- Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083-91. [Crossref] [PubMed]
- Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092-100. [Crossref] [PubMed]
- Mojadidi MK, Gevorgyan R, Tobis JM. Device Closure of Patent Foramen Ovale or Medical Therapy for Cryptogenic Stroke: The CLOSURE I Trial. In: Amin Z, Tobis J, Sievert H, et al. editors. Patent Foramen Ovale. London: Springer, 2015:173-9.
- Mojadidi MK, Christia P, Salamon J, et al. Patent foramen ovale: Unanswered questions. Eur J Intern Med 2015;26:743-51. [Crossref] [PubMed]
- Khattab AA, Meier B. The PC Trial: An Effective Treatment Not Demonstrating Effective Power. In: Amin Z, Tobis J, Sievert H, et al. editors. Patent Foramen Ovale. London: Springer, 2015:185-8
- Saver JL, Carroll JD, Thaler DE, et al. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med 2017;377:1022-32. [Crossref] [PubMed]
- Kent DM, Dahabreh IJ, Ruthazer R, et al. Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol 2016;67:907-17. [Crossref] [PubMed]
-
AMPLATZER PFO Occluder - P120021 - Mas JL, Derumeaux G, Guillon B, et al. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med 2017;377:1011-21. [Crossref] [PubMed]
- Søndergaard L, Kasner SE, Rhodes JF, et al. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med 2017;377:1033-42. [Crossref] [PubMed]
- Mojadidi MK, Mahmoud AN, Elgendy IY. Percutaneous patent foramen ovale closure for cryptogenic stroke: learning from clinical trial and error. J Thorac Dis 2017; [Epub ahead of print].
- Mojadidi MK, Elgendy AY, Elgendy IY, et al. Transcatheter Patent Foramen Ovale Closure After Cryptogenic Stroke: An Updated Meta-Analysis of Randomized Trials. JACC Cardiovasc Interv 2017;10:2228-30. [Crossref] [PubMed]
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478-86. [Crossref] [PubMed]
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467-77. [Crossref] [PubMed]
Cite this article as: Mojadidi MK, Elgendy IY, Cutting WB, Mojaddedi S, Mahmoud AN. Percutaneous patent foramen ovale closure for cryptogenic ischemic stroke: is it time for new guidelines? AME Med J 2017;2:173.

