
Metabolic inflammatory syndrome: a novel concept of holistic integrative medicine for management of metabolic diseases
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and the cluster of its risk components due to metabolic imbalance has been labeled variously as Syndrome X or the Insulin Resistance Syndrome, and then the metabolic syndrome (MS) (1). Evidences have indicated that insulin resistance may be the common etiological factor for these risk components including obesity, hypertension, dyslipidemia and hyperglycemia (2). However, there appears to be heterogeneity in the strength of the insulin resistance relationship with different risk components between or within the populations (2).
Chronic low-grade inflammation in metabolic organisms and tissues is known as metabolic inflammation or ‘meta-inflammation’ (3). Activation of inflammatory pathways and immune system has been observed in adipose tissue, liver, muscle, pancreas, artery and brain and participates in the pathogenesis and progression of obesity and overweight, non-alcohol fatty liver disease (NAFLD), hyperglycemia and atherosclerosis (4-7). Thus, meta-inflammation may be another way to explore the interrelationship of these metabolic diseases to supplement the limitation of insulin resistance.
Epidemic evidence is accumulating that a person with hyperglycemia is more likely to have at least one or more of the other metabolic diseases (8-10) and anti-inflammatory interventions can improve more than one metabolic disease meanwhile (11,12). Metabolic diseases are gathered by the term of MS according to their association with CVD (2). However, some of them, such as atherosclerosis and NAFLD, are not included in MS (2) but linked by meta-inflammation (13). Therefore, we proposed a new concept of metabolic inflammatory syndrome (MIS) to gather the metabolic inflammatory diseases and hypothesized the definition of MIS as a person with at least two or more of the following components: atherosclerosis, type 2 diabetes (T2D), obesity and overweight and NAFLD (14).
To clarify the clinical value of the concept of MIS, we conducted a multi-center cross-sectional study in inpatients with T2D. Here we present the analysis results of MIS detective rate and its risk for coronary heart disease (CHD).
Methods
Study population
The medical centers included in our study were Huashan Hospital Affiliated to Fudan University, No. 3 People’s Hospital Affiliated to Jiaotong University, No. 6 People’s Hospital Affiliated to Jiaotong University, No. 1 People’s Hospital Affiliated to Jiaotong University and the First Affiliated Hospital of Harbin Medical University. All the hospitals above used the electronic medical records systems to record the history of diseases and clinical data of the inpatients. We enrolled the adults reporting a diagnosis of T2D, according to World Health Organization, at least 18 years of age and having registered for admission to Department of Endocrinology in these hospitals from January 1, 2013 to January 1, 2015. Participants were excluded if they had a history of alcohol consumption, chronic immune disease, acute infection or severe state during hospitalization. A total of 5,007 inpatients were included and 4,711 participants were eligible for our analysis. The study protocol was approved by the institutional review boards of Huashan Hospital Affiliated to Fudan University. The first, second and last authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Assessment of MIS
Because all the participants had T2D, a participant who had one or more of other MIS components below was considered to have MIS: atherosclerosis, obesity and overweight (obesity/overweight), and NAFLD. As peripheral artery disease was the marker of systemic atherosclerosis (15), in this study atherosclerosis was assessed by continuous-wave Doppler ultrasound blood flow measurements and duplex ultrasound measurements for carotid artery and lower extremity artery (16). NAFLD was screened by ultrasound too. In our study, NAFLD was defined as ultrasonographic evidence of hepatic steatosis with no causes for secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders. Obesity and overweight was assessed using body mass index (BMI) which was calculated as weight in kilograms divided by height in meters squared. Weight and height were measured at morning before breakfast. Obesity was defined as BMI of 30 or more and overweight was defined as BMI of 25 to 30.
Assessment of MS
In our study, MS was diagnosed using the criteria of Chinese Diabetes Society (CDS) (17). For all the patients were with T2D, a patient who had two or more of the following MS components was considered to have MS: hypertension and dyslipidemia, obesity and overweight (2). Hypertension was assessed by the measurement of blood pressure and it referred to at least one of the following: systolic blood pressure of 140 or more; diastolic blood pressure of 90 or more; history of hypertension. Dyslipidemia here referred to at least of the following: triglycerides (TG) of 1.7 mmol/L or more; high-density lipoprotein cholesterol (HDL-C) less than 1.0 mmol/Lin women and 0.9 mmol/L in men. For hyperuricemia and albuminuria were candidates of MS mentioned in other guidelines or literature (2,18), we also assessed them here. Hyperuricemia was defined as serum uric acid (UA) of 360 mol/L or more in women and 420 mol/L or more in men. Albuminuria was defined as albuminuria creatinine ratio (ACR) of spot urinary sample in the morning of 30 mg/g or more. Results of UA and ACR were recorded by the electronic medical records systems of hospitals.
Assessment of covariables
Medical records of age, gender, cigarette smoking, duration of T2D, history of hypertension and atherosclerotic CHD and results of HbA1c were collected by browsing the electronic medical records systems.
Statistic analysis
Differences of numerical variables between participants with MIS and no MIS were assessed by using one-way ANOVO analysis. Differences of proportions between participants with MIS and no MIS were tested by means of chi-square analysis. Odds ratios of MIS and MS and their components to predict CHD were estimated by binary logistic analysis. A P value of less than 0.05 was considered to indicate a statistical significance. All statistical analyses were performed using the computerized statistical package SPSS for Windows, version 16.0 (SPSS Inc., IBM, USA).
Results
Study participants
For the 4,711 participants included in our analysis, the mean age was 56 years (range, 18 to 90 years). The characteristics of participants were presented in Table 1, stratified by gender and MIS categories. Compared with participants with no MIS, participants with MIS were older and had higher percentage of hypertension and history of CHD in both male and female subgroups. Participants with MIS had longer duration of T2D and higher level of HbA1c than those with no MIS for male subgroup but not female subgroup. Participants with MIS had higher percentage of smoking and dyslipidemia than those with no MIS for female subgroup but not male subgroup. HOMAIR has no difference between participants with and without MIS.
Table 1
| Characteristic | Male participants | Female participants | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| With MIS | With no MIS | P value | With MIS | With no MIS | P value | ||||
| No. of participants | 2,674 | 85 | – | 1,860 | 92 | – | 4,711 | ||
| Age (year) | 54.3±13.0 | 47.3±14.3 | <0.001 | 59.9±11.6 | 51.7±14.9 | <0.001 | 56.4±12.9 | ||
| T2D duration (year) | 7.5±6.8 | 6.0±6.0 | 0.039 | 9.1±7.4 | 7.8±7.0 | 0.097 | 8.1±7.1 | ||
| HbA1c (%) | 8.8±2.1 | 9.7±2.6 | <0.001 | 8.7±2.2 | 8.8±2.5 | 0.705 | 8.8±2.2 | ||
| HOMAIR(mmol·mU·L−2) | 7.7±16.9 | 8.9±12.7 | 0.609 | 8.3±15.3 | 6.4±13.3 | 0.362 | 7.9±16.2 | ||
| Smoking (%) | 46.3 | 40.0 | 0.150 | 5.8 | 1.1 | 0.033 | 29.3 | ||
| Hypertension (%) | 64.0 | 34.1 | <0.001 | 67.4 | 45.7 | <0.001 | 64.4 | ||
| Dyslipidemia (%) | 35.6 | 28.4 | 0.122 | 42.7 | 31.7 | 0.031 | 38.2 | ||
| Albuminuria (%) | 15.7 | 18.3 | 0.346 | 19.1 | 16.7 | 0.368 | 17.1 | ||
| Hyperuricemia (%) | 28.0 | 35.4 | 0.093 | 30.9 | 28.1 | 0.332 | 29.3 | ||
| CHD (%) | 15.3 | 1.2 | <0.001 | 22.5 | 7.6 | <0.001 | 17.7 | ||
MIS, metabolic inflammatory syndrome; T2D, type 2 diabetes; CHD, coronary heart disease.
Detective rate of MIS and its components
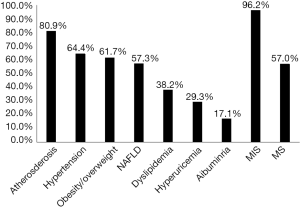
Among the 4,711 participants who had T2D, detective rate of MIS was 96.2%, comparing with 57.0% for MS. Atherosclerosis had the highest detective rate of 80.9% among other components of MIS except T2D. Hypertension had the highest detective rate of 64.4% among the components of MS and slightly higher than obesity/overweight (with detective rate of 61.7%) and NAFLD (with detective rate of 57.3%) (Figure 1).
According to the numbers of MIS components except T2D, MIS had three types of combinations and details were listed in Table 2. Combination with two components had the highest detective rate of 40.5% among the three types of combinations. The combination ‘AS + obesity/overweight’ had the highest detective rate of 17.7% among all the combinations.
Table 2
| Combination of MIS components in participants with T2D | No. of participants | Detective rate (%) |
|---|---|---|
| MIS | 4,535 | 96.2 |
| Combinations with other mere one component | 1,140 | 24.2 |
| AS | 800 | 17.0 |
| Obesity/overweight | 205 | 4.4 |
| NAFLD | 135 | 2.9 |
| Combinations with other two components | 1,910 | 40.5 |
| AS + obesity/overweight | 832 | 17.7 |
| Obesity/overweight + NAFLD | 384 | 8.2 |
| AS + NAFLD | 694 | 14.7 |
| Combinations with other three components (AS + obesity/overweight + NAFLD) | 1,485 | 31.5 |
MIS, metabolic inflammatory syndrome; T2D, type 2 diabetes; AS, atherosclerosis; NAFLD, non-alcoholic fatty liver disease.
Risk of MIS and its components for CHD
Results indicated that MIS was a risk factor for CHD in all the participants who had T2D, with a greater odds ratio than MS [MIS: odds ratio, 4.71; 95% confidence interval (CI), 2.31 to 9.62; P<0.001; MS: odds ratio, 1.61; 95% CI, 1.38 to 1.88; P<0.001]. After adjustment for age, duration of T2D, HbA1c and smoking, MIS was also a risk factor for CHD in all the participants, with its odds ratio (MIS: odds ratio, 3.35; 95% CI, 1.62 to 6.93; P<0.001) less than that before adjustment but greater than that of MS after adjustment (MS: odds ratio, 1.35; 95% CI, 1.14 to 1.59; P<0.001) (Figure 2).

Among the components of MIS, atherosclerosis and obesity/overweight were independent risk factors for CHD after adjustment for age, T2D duration, HbA1c and smoking, as did hypertension, a component of MS. Comparing the components of MIS and MS, atherosclerosis had the greatest odds ratio before and after adjustment (no adjustment: odds ratio, 3.58; 95% CI, 2.72 to 4.70; P<0.001; After adjustment: odds ratio, 2.21; 95% CI, 1.65 to 2.97; P<0.001). By contrast, results indicated that NAFLD seemed to play a protective role for CHD before adjustment (odds ratio, 0.84; 95% CI, 0.72 to 0.97; P=0.020) but the result had no statistical significance after adjustment (P>0.05). The result of odds ratio of dyslipidemia for CHD had no statistical significance before or after adjustment (P>0.05) (Figure 2).
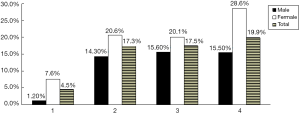
The detective rates of CHD within the patients with one, two, three and four MIS components were 4.5%, 17.3%, 17.5% and 19.9%, respectively. The result indicated that detective rate of CHD increased with the number of MIS components. This trend was also observed in the male subgroup and female subgroup (Figure 3). In other words, if one person had more MIS components, he was more likely to have CHD.
Discussion
Our analyses of data from a multi-center cross-sectional study show the clinical value of MIS. In patients with T2D, all the other metabolic inflammatory diseases defined as MIS components here, including atherosclerosis, obesity and overweight and NAFLD, have high detective rates (more than 50%). Combinations of these three components also have high detective rates, especially the combinations with two components. Thus, a person with T2D has a likelihood of more than 90% to have one of these combinations (defined as MIS here). Our findings support the phenomenon that a metabolic disease always coexists or accompanies with the other metabolic diseases (8-10).
This phenomenon is used to be explained by insulin resistance (1). Recently, the association of chronic low-grade inflammation and metabolic disorders has been established. A cluster of metabolic diseases such as obesity, T2D, atherosclerosis and NAFLD are inflammatory diseases (3-7). They are various conditions associated with meta-inflammation and share some common dysfunctional pathways (19). Meta-inflammation is initiated by damage associated molecular patterns (DAMPs) such as free saturated fatty acids (FFAs) due to unhealthy lifestyle and pathogen associated molecular patterns (PAMPs) such as bacterial lipopolysaccharide (LPS) absorbed from gut microbiota. DAMPs and PAMPs bind classical pattern recognition receptors (PRRs) including toll-like receptors (TLRs) complex on the surface of immune cells to activate inflammatory pathways together with the other factors such as microRNAs and interferon (20-22). Macrophage plays a central role in meta-inflammation. Its activated M1-type promotes inflammation by secreting proinflammatory cytokines and ROS, while its M2-type may prevent inflammation and modulate immune responses (23). Macrophage polarity, which means that an abundant of macrophages shift towards M1 and leads to elevated M1/M2 ratio, in the metabolic organs and tissues, will be induced by increasing FFAs, LPS, interferon or some microRNAs and promotes the formation and progression of metabolic diseases including obesity, T2D, atherosclerosis and NAFLD (20-25) (Figure 3). Thus the phenomenon mentioned above may be explained by meta-inflammation which appears to be another important nexus between these metabolic diseases and MIS is a term for this relationship.
The gathering of metabolic diseases makes sense. A typical example is that many metabolic diseases are the risk factors in the development of CHD. Some metabolic risk factors such as obesity and overweight, dyslipidemia and hyperglycemia, are defined as the components of MS together with the other risk factors including hypertension and albuminuria (1). However, some metabolic risk factors such as atherosclerosis are not included in the definition of MS. In our study, MIS includes atherosclerosis as the component. Our results show that MIS and atherosclerosis, as well as hypertension, obesity and overweight and MS, are independent risk factors of CHD. These findings not only confirm the traditional CHD risk factors, but also represent the important significance of metabolic diseases gathered by inflammation (MIS components) for CHD. Moreover, our study shows that detective rate of CHD increases with the number of MIS components. Thus it makes sense that a person with T2D or any other MIS component needs to examine the other MIS components and CHD.
Atherosclerosis should be highlighted here. In our study, atherosclerosis has the highest detective rate and the greatest odds ratio for CHD among all the MIS and MS components, contributing to the characteristic of MIS. Our study tests plaque and thickness of intima of carotid artery and lower extremity artery by ultrasound to diagnoses atherosclerosis. Ultrasound is an invasive test and easy for application so that examination of atherosclerosis is suitable for the primary screening of MIS in patients with T2D and is important for the diagnosis and treatment of atherosclerosis itself.
In our study, MIS is a concept different from MS though they have some common components. Compared with MS, the strengths of MIS include the higher detective rate and the greater odds ratio for CHD. All the components included by different definitions of MS have been tested here. Hypertension is an independent risk factor for CHD (1), associated with inflammation (26) and highly detective in patients with T2D. However, the mechanisms of hypertension are complicated (27) and it is ambiguous to define hypertension as a metabolic inflammatory disease or not for its complicated mechanisms, so it is not included in MIS components in our study. The other MS components, except hypertension and obesity and overweight, all have the detective rates of lower than 50%.
Some results of the study have not been explained yet, including that dyslipidemia is not the risk factor of CHD and that NAFLD presents to be a protective factor for CHD though it has no statistical significance after adjustment.
Limitation of this study includes the study populations. All the study participants are inpatients and have T2D in China, so the conclusions here may be only suitable for Chinese inpatients with T2D. But our study is still at a preliminary stage so that improvements can be made in the future. Information on history of disease was obtained from the electronic medical records so the memory bias was inevitable.
In conclusion, our findings present that MIS may be a new concept for management of metabolic diseases. MIS has a high detective rate and appears to be an independent risk factor of CHD in patients with T2D. A person who has one of the MIS components should be examined the other MIS components. Atherosclerosis, contributing to the characteristics of MIS, is suitable for screening MIS. As MIS is based on meta-inflammation, MIS may be useful for researches of inflammatory mechanisms of the conventional metabolic therapies such as aerobic exercise and metformin, and exploring of the metabolic therapies targeting inflammation (28,29). The molecules that suppress macrophage polarity, such as GLP-1 and metformin, is potential for metabolic treatment (12,30). We defined four diseases as the components of MIS here. It seems that MIS is a novel concept of holistic integrative medicine for management of metabolic diseases.
Acknowledgements
Funding: This research was supported by the National Natural Science Foundation of China (No. 81471057, 81270902, 81030014).
Footnote
Provenance and Peer Review: This article was commissioned by AME Medical Journal for the series “Holistic Integrative Medicine”. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.03.11). The series “Holistic Integrative Medicine” was commissioned by the editorial office without any funding or sponsorship. Dr. Daiming Fan served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the institutional review boards of Huashan Hospital Affiliated to Fudan University (KY2016-320). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med 1993;44:121-31. [Crossref] [PubMed]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1 diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [Crossref] [PubMed]
- Cildir G, Akıncılar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med 2013;19:487-500. [Crossref] [PubMed]
- Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature 2017;542:177-85. [Crossref] [PubMed]
- Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017;17:306-21. [Crossref] [PubMed]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98-107. [Crossref] [PubMed]
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med 1999;340:115-26. [Crossref] [PubMed]
- Hu DY, Pan CY, Yu JM. The relationship between coronary artery disease and abnormal glucose regulation in china: The China Heart Survey. Eur Heart J 2006;27:2573-9. [Crossref] [PubMed]
- Wild SH, Morling JR, McAllister DA, et al. Type 2 diabetes, chronic liver disease and hepatocellular cancer: a national retrospective cohort study using linked routine data. J Hepatol 2016;64: [PubMed]
- Yoon KH, Lee JH, Son HY, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681-8. [Crossref] [PubMed]
- Song R, Peng W, Xiao RP, et al. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature 2013;494:375-9. [Crossref] [PubMed]
- Li R, Shen Q, Wu N, et al. MiR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine 2018;60:73-82. [Crossref] [PubMed]
- Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600-7. [Crossref] [PubMed]
- Hu R, Xie Y, Lu B, et al. High Exploratory Rate of “Metabolic Inflammatory Syndrome (MIS)” In The patients with Type 2 Diabetes. Chin J Endocrinol Metab 2016;32:27-32.
- Olin JW, Allie DE, Masoudi FA, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease. Circulation 2010;122:2583-618. [Crossref] [PubMed]
- Rooke TW, Hirsch AT, Zierler RE, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations). J Am Coll Cardiol 2013;61:1555-70. [Crossref] [PubMed]
- Chinese diabetes society metabolic syndrome collaboration group. Chinese diabetes society recommendations about metabolic syndrome. Chin J Diabetes 2004;12:156-61.
- Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 2013;25:210-6. [Crossref] [PubMed]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363-74. [Crossref] [PubMed]
- McNelis JC, Olefsky JM. Macrophages, Immunity, and Metabolic Disease. Immunity 2014;41:36-48. [Crossref] [PubMed]
- Chen CZ, Schaffert S, Fragoso R, et al. Regulation of immune responses and tolerance: the microRNA perspective. Immunol Rev 2013;253:112-28. [Crossref] [PubMed]
- Rocha VZ, Folco EJ, Sukhova G, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 2008;103:467-76. [Crossref] [PubMed]
- Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab 2013;15:152-8. [Crossref] [PubMed]
- Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009;51:212-23. [Crossref] [PubMed]
- Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N Engl J Med 2005;352:1685-95. [Crossref] [PubMed]
- Idris-Khodja N, Mian MO, Paradis P, et al. Dual opposing roles of adaptive immunity in hypertension. Eur Heart J 2014;35:1238-44. [Crossref] [PubMed]
- Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116:991-1006. [Crossref] [PubMed]
- Lancaster GI, Febbraio MA. The immunomodulating role of exercise in metabolic disease. Trends Immunol 2014;35:262-9. [Crossref] [PubMed]
- Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54:1566-72. [Crossref] [PubMed]
- Shiraishi D, Fujiwara Y, Komohara Y, et al. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun 2012;425:304-8. [Crossref] [PubMed]
Cite this article as: Hu R, Xie Y, Lu B, Li Q, Chen F, Li L, Hu J, Huang Y, Li Q, Ye W, Li R, Liu N, Huang J, Zhang Z, Zhou L, He M, Fan W, Liu J, Wen J, Chen L, Yang Y, Li Y, Fan D, Zhu X. Metabolic inflammatory syndrome: a novel concept of holistic integrative medicine for management of metabolic diseases. AME Med J 2018;3:51.

