
A nucleotide-binding oligomerization domain for innate immunity and reproduction
Exposure to the various environmental microbial pathogens is a constant threat to mammals. As a rapid mobilized first line defense, innate immunity is of critical importance for mammalian cells to maintain their normal activities. One of the key features of innate immune response is to distinguish non-self from self cues and thus initiates downstream defense responses including releasing cytokines, activation and recruitment of immune cells, etc. Identification of pathogenic cues relies on a special group of germline-encoded surface and intracellular proteins, called pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide binding domain, leucine-rich repeat (LRR)-containing [or nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs)], RIG-I like receptors (RLRs), and the AIM2-like receptors (ALRs) (1). Recognition of foreign molecular cues will trigger a cascade of events resulted in activation of immune related genes and rapid releasement of associated cytokines.
However, foreign molecular cues are not always from environmental pathogens. A couple of unique situations in mammals exist where foreign cues are not a threat and innate immunity shall be repressed, such as fertilization and pregnancy. Dysregulation of innate immune regulation thus could have a significant impact on human fertility. In fact, nearly one-half of idiopathic infertility cases are believed to have a genetic cause (2) and many of the identified infertility associated genes are immune related (3). However, the underlining molecular mechanism for human infertility is still largely unknown.
NOD, LRR, and pyrin domain containing proteins (NLRPs) are members of NLRs protein family (4). Previous studies focused on their roles in apoptotic and inflammatory signaling pathways via the formation of an inflammasome and activation of caspases in innate immunity (5-7). However, more recent researches have revealed their roles in mammalian reproduction (Table 1).
Table 1
| Gene | Aliases | Expression location | Function | Reference |
|---|---|---|---|---|
| NLRP2 | CLR19.9, NALP2, NBS1, PAN1, PYPAF2 | Ovary | Associated with imprinting in BWS in human; NLRP2 knockdown embryo block at 2-cell stage in mouse | Zhang et al., 2008 (8); Meyer et al., 2009 (9); Hui et al., 2012 (10) |
| NLRP4 | CLR19.5, CT58, NALP4, PAN2, PYPAF4, RNH2 | Ovary; testis | May play essential role during zygotic genome activation in the mouse. NLRP4 knockdown embryo block at 2–8 cells stage | Tian et al., 2009 (11); Chang et al., 2013 (12); Peng et al., 2014 (13) |
| NLRP5 | Mater, NALP5, OP1, PAN11 | Ovary | The NLRP5-null mouse stops the development of the embryo; maternal depletion of NLRP5 blocks early embryogenesis in monkey; mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans NLRP5 mediates mitochondrial function in mouse oocytes and embryos | Tong et al., 2000 (14); Fernandes et al., 2012 (15); Docherty et al., 2015 (16) |
| NLRP7 | CLR19.4, HYDM, NALP7, NOD12, PAN7, PYPAF3 | Ovary | Mutations cause abnormal embryo development in human; NLRP7 mutations are at risk for reproductive wastage in woman; maternal heterozygous NLRP7 variant results in recurrent reproductive failure and imprinting disturbances in the offspring | Murdoch et al., 2006 (17); Qian et al., 2007 (18); Monk et al., 2017 (19) |
| NLRP8 | CLR19.2, NALP8, NOD16, PAN4 | Lost in rodents; germ-cell-specific in cattle | – | Tian et al., 2009 (11) |
| NLRP9 | CLR19.1, NALP9, NOD6, PAN12 | Ovary; testis | – | Hamatani et al., 2004 (20); Dade et al., 2004 (21); Ponsuksili et al., 2006 (22); Tian et al., 2009 (11) |
| NLRP11 | CLR19.6, NALP11, NOD17, PAN10, PYPAF6, PYPAF7 | Primate-specific | – | Tian et al., 2009 (11) |
| NLRP13 | ClR19.7, NALP13, NOD14, PAN13 | Ovary; testis; lost in rodents | – | Tian et al., 2009 (11) |
| NLRP14 | CLP11.2, GC-LRR, NALP14, NOD5, PAN8, NALP-IOTA | Ovary; testis | Knockdown mouse stops the development of the embryo; associated with human spermatogenesis failure; negatively regulates cytosolic nucleic acid sensing to promote fertilization | Gu et al., 2002 (23); Hamatani et al., 2004 (20); Westerveld et al., 2006 (24); Abe et al., 2017 (25) |
NLRP, nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain containing proteins.
In mouse, the first identified mammalian maternal effect gene in this family is NLRP5, which encodes mRNA required for successful development of a fertilized oocyte (14). Additionally, expression analysis of NLRP genes in the human and macaque monkeys (Macaca mulatta) has shown that most if not all NLRP genes are expressed specifically in primate gametes and early embryos, suggesting a general role of NLRPs in primate preimplantation development (18,26), as well as their involvement in innate immunity.
Nlrp14 gene is one of the key members in NLRP family. NLRP14 protein typically contains a NACHT domain, a NACHT-associated domain (NAD), a C-terminal LRR, and 14 N-terminal pyrin domain (PYD). It is also known as NALP14, NOD5, GC-LRR, Nalp-iota, PAN8, and CLR11.2. Expression of NLRP14 has been confirmed through an unbiased proteomics approach in oocytes (27). Its mRNA transcript level appeared to decrease with age, coinciding with reduced fertility (20). These evidences suggest that NLRP14 may play a role in female reproduction, although detailed mechanism remains to be explored.
Meanwhile, northern analysis of multiple tissues with a NLRP14 specific probe indicated that NLRP14 was exclusively expression in human adult testis (24) rather than ovary, which was also confirmed in mouse tissues (20,21). Immuno-histology analysis revealed that NLRP14 showed a clear signal in the cytoplasm of a dark spermatogonia, which is reserve as stem cell, mid and late pachytene spermatocytes and spermatids (24). Sertoli cells also showed some cytoplasmic staining for NLRP14 (24). It seems that NLRP14 has the roles in the most types of the male germ cell, particularly in the spermatogonia, or even in primordial germ cell.
Moreover, after a mutation screen of the NLRP14 gene in 157 men with azoospermia or sever oligozoospermia by direct sequencing, the researchers identified 25 sequence variants in total; 1 nonsense mutation, 14 missense mutation, 6 silent mutation and 4 intronic variants (24). One of these mutation was an early stop codon mutation (p.K108X), this A to T change in exon 2 introduced a stop codon at amino acid position 108, and it would lead to lack the functional NACHT and LRR domains (24). Accordingly, NLRP14 seems to be very important for spermatogenesis, but the specific molecular mechanism is unknown yet.
Another important process in reproduction is fertilization. Fertilization is the fusion of gametes from parents to initiate the development of a new embryo. For oocytes, nucleic acids from sperms is a non-self signal that may trigger cytosolic PRRs and initiate innate immune response. Therefore, embryos must have a regulatory network in place to suppress innate immune activation to achieve successful fertilization. In a recent study by Abe et al., NLRP14 was identified as a key player in regulating innate immune response in oocyte and negatively regulates cytosolic nucleic acid sensing to promote fertilization (Figure 1) (25).
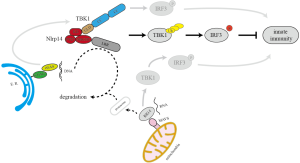
In this study, evidence was shown that NLRP14 could achieve the highest suppression of STING signaling mediated by cGAS, an important cytosolic DNA sensor (25). Additionally, loss- and gain-of-function experiments in 293T cell revealed that NLRP14 might interact with a kinase called TBK1 for ubiquitination and degradation to negatively regulates cytosolic sensing of DNA and RNA (25). Ectopic expression of the K180X allele of NLRP14, which has identified in sterile men with spermatogonia failure (24), resulted in reduced inhibition of TBK1-mediated signaling (25). This allele has an average frequency of 1.7% in the human population and a minor allele frequency of 3% in East Asian and admixed American populations (28), suggesting that the infertility which caused by this kind of mutation may have a wide range of impact.
Furthermore, The sub-clone version of this allele, just containing sequence for amino acid from 1 to 108, showed further enhancement of TBK1-mediated IFN- and NF-B activation (25). Another published data showed that the excess amount of type I IFN signaling disrupts seminiferous tubules in mice, leading to a loss of germ cells and infertility (29). These reports indicated that some NLRP14 mutations might cause spermatogenesis failure through inappropriate innate immune responses in testis.
In summary, NLRP14 seems to be very important for both female and male reproduction. This study was the first report towards understanding the function of NLRP14 in innate immune regulation and its role in human reproduction. Meanwhile, as many of these experiments were done in established non-germline cell lines, more efforts are warranted to explore the function of NLRP14 in vivo and whether it could have different interacting partners in male gametes and oocytes. Nevertheless, the finding has set up a solid ground that there is a NOD between innate immunity and reproduction.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Ziwei Li (Department of Surgery, The First Hospital Affiliated to Kunming Medical School, Kunming, China).
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.03.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brubaker SW, Bonham KS, Zanoni I, et al. Innate Immune Pattern Recognition: A Cell Biological Perspective. Annu Rev Immunol 2015;33:257-90. [Crossref] [PubMed]
- Zorrilla M, Yatsenko AN. The Genetics of Infertility: Current Status of the Field. Curr Genet Med Rep 2013;1. [PubMed]
- Brazdova A, Senechal H, Peltre G, et al. Immune Aspects of Female Infertility. Int J Fertil Steril 2016;10:1-10. [PubMed]
- Ting PY, Lovering RC, Alnemri ES, et al. The NLR Gene Family: A Standard Nomenclature. Immunity 2008;28:285-7. [Crossref] [PubMed]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature 2006;442:39. [Crossref] [PubMed]
- Martinon F, Gaide O, Pétrilli V, et al. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol 2007;29:213. [Crossref] [PubMed]
- Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol 2003;4:95-104. [Crossref] [PubMed]
- Zhang P, Dixon M, Zucchelli M, et al. Expression analysis of the NLRP gene family suggests a role in human preimplantation development. PLoS One 2008;3:e2755 [Crossref] [PubMed]
- Meyer E, Lim D, Pasha S, et al. Germline mutation in NLRP2 (NALP2) in a familial imprinting disorder (Beckwith-Wiedemann Syndrome). PLoS Genetics 2009;5:e1000423 [Crossref] [PubMed]
- Hui P, Chang B, Lu C, et al. Nlrp2, a Maternal Effect Gene Required for Early Embryonic Development in the Mouse. PLoS One 2012;7:e30344 [Crossref] [PubMed]
- Tian X, Pascal G, Monget P. Evolution and functional divergence of NLRP genes in mammalian reproductive systems. BMC Evol Biol 2009;9:202. [Crossref] [PubMed]
- Chang BH, Zhang Y. Developmental expression and possible functional roles of mouse Nlrp4e in preimplantation embryos. In Vitro Cell Dev Biol Anim 2013;49:548-53. [Crossref] [PubMed]
- Peng H, Zhang W, Xiao T, et al. Nlrp4g is an oocyte-specific gene but is not required for oocyte maturation in the mouse. Reprod Fertil Dev 2014;26:758-68. [Crossref] [PubMed]
- Tong ZB, Gold L, Pfeifer KE, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nat Genet 2000;26:267-8. [Crossref] [PubMed]
- Fernandes R, Tsuda C, Perumalsamy AL, et al. NLRP5 mediates mitochondrial function in mouse oocytes and embryos. Biol Reprod 2012;86:1-10. [PubMed]
- Docherty LE, Rezwan FI, Poole RL, et al. Mutations in NLRP5 are associated with reproductive wastage and multilocus imprinting disorders in humans. Nat Commun 2015;6:8086. [Crossref] [PubMed]
- Murdoch S, Djuric U, Mazhar B, et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat Genet 2006;38:300-2. [Crossref] [PubMed]
- Qian J, Deveault C, Bagga R, et al. Women heterozygous for NALP7/NLRP7 mutations are at risk for reproductive wastage: report of two novel mutations. Hum Mutat 2007;28:741. [Crossref] [PubMed]
- Monk D, Sanchezdelgado M, Fisher R. NLRPs, the subcortical maternal complex and genomic imprinting. Reproduction 2017;154:R161-70. [Crossref] [PubMed]
- Hamatani T, Falco G, Carter MG, et al. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 2004;13:2263-78. [Crossref] [PubMed]
- Dade S, Callebaut IA, Bontoux M, et al. In silico identification and structural features of six new genes similar to MATER specifically expressed in the oocyte. Biochem Biophys Res Commun 2004;324:547-53. [Crossref] [PubMed]
- Ponsuksili S, Brunner RM, Goldammer T, et al. Bovine NALP5, NALP8, and NALP9 genes: assignment to a QTL region and the expression in adult tissues, oocytes, and preimplantation embryos. Biol Reprod 2006;74:577-84. [Crossref] [PubMed]
- Gu X, Vander VK. DIVERGE: phylogeny-based analysis for functional-structural divergence of a protein family. Bioinformatics 2002;18:500-1. [Crossref] [PubMed]
- Westerveld GH, Korver CM, van Pelt AM, et al. Mutations in the testis-specific NALP14 gene in men suffering from spermatogenic failure. Hum Reprod 2006;21:3178-84. [Crossref] [PubMed]
- Abe T, Lee A, Sitharam R, et al. Germ-Cell-Specific Inflammasome Component NLRP14 Negatively Regulates Cytosolic Nucleic Acid Sensing to Promote Fertilization. Immunity 2017;46:621-34. [Crossref] [PubMed]
- Mcdaniel P, Wu X. Identification of oocyte-selective NLRP genes in rhesus macaque monkeys (Macaca mulatta). Mol Reprod Dev 2009;76:151-9. [Crossref] [PubMed]
- Wang S, Kou Z, Jing Z, et al. Proteome of mouse oocytes at different developmental stages. Proc Natl Acad Sci U S A 2010;107:17639-44. [Crossref] [PubMed]
- Consortium TGP. A global reference for human genetic variation. Nature 2015;526:68-74. [Crossref] [PubMed]
- Ulusoy E, Cayan S, Yilmaz N, et al. Interferon alpha-2b may impair testicular histology including spermatogenesis in a rat model. Arch Androl 2004;50:379-85. [Crossref] [PubMed]
Cite this article as: Yin Y, Shan C, Li Z. A nucleotide-binding oligomerization domain for innate immunity and reproduction. AME Med J 2018;3:52.

