
Relationship among biomarkers of iron metabolism and severity of underlying nonalcoholic steatohepatitis (NASH)
Introduction
Changes in eating habits are associated with increased prevalence of obesity, type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS) and in this setting, non-alcoholic fatty liver disease (NAFLD) has been highlighted as the most common worldwide cause of chronic liver disease (1-3). The global prevalence of NAFLD ranges from 6.3% to 33%, with an average of 20% in the general population, based on studies using various diagnostic methods (4). Among the most advanced stages of NAFLD, nonalcoholic steatohepatitis (NASH) is defined by the presence of hepatic steatosis associated with inflammatory activity, hepatocyte ballooning and necrosis with several degrees of fibrosis, leading to hepatic complications, like cirrhosis and increased risk for hepatocellular carcinoma (HCC).
NAFLD is usually asymptomatic and diagnosed accidentally by imaging tests or due to increased levels of aminotransferases (5-8). The histological diagnosis of NAFLD through liver biopsy is the most reliable diagnostic method and although invasive and expensive, it is the gold standard to show the extent of steatosis, inflammation and hepatic fibrosis (5,9,10).
The liver is the major storage organ of iron and produces transferrin and hepcidin, which regulate iron metabolism. Iron homeostasis is ultimately controlled at the absorption level in the duodenum. The discovery of hepcidin and the hemochromatosis gene (HFE) have made us very clear about the metabolism and overload of iron(11). Overload of hepatic iron and its correlation with chronic liver disease has been the subject addressed in many studies (12-14).
Hepatic iron load in NASH and other chronic liver diseases may be associated with the risk of toxicity, acting as a comorbidity factor, associated with fat, hepatitis virus or alcohol. It has been pointed as a fuel for the oxidative stress involved in fibrogenesis and carcinogenesis (15).
The accumulation of sinusoidal iron may be considered an important risk factor in the progression of chronic liver diseases and/or HCC (13). As there is evidence of abnormalities of iron metabolism in the pathogenesis and progression of NAFLD, we evaluated the relationship of iron metabolism markers with the severity of NASH in a cohort of adult obese individuals.
Methods
A total of 88 adult subjects attended at the unit of Gastroenterology and Hepatology of Botucatu Clinical Hospital (UNESP) between 2010 and 2014 were included in the study. The inclusion criteria were: age between 18 and 80 years old, both genders, body mass index (BMI) consistent with obesity (16) and the diagnosis of NASH confirmed by liver biopsy (17). Subjects with a history of alcohol intake greater than 30 g/day, positive serology for hepatitis B or C, autoimmune hepatitis (18), presence of potentially hepatotoxic drugs with steatosis as the predominant lesion and confirmed liver diseases of other causes were excluded from the study. The study was approved by the local Hospital Ethical Committee.
The subjects were submitted to a complete clinical evaluation, with history, nutritional status assessment and physical examination, including blood pressure, BMI and waist circumference (WC) measurement (19).
After a fasting night, venous blood was collected for laboratory tests: albumin, total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), total cholesterol, HDL-cholesterol (high density lipoprotein), LDL-cholesterol (low density lipoprotein), triglycerides (TG), fasting glycemia and basal insulin. The level of insulin resistance was determined by the homeostatic evaluation model (HOMA) (20).
The iron metabolism biomarkers analysed included serum levels of transferrin, ferritin, serum iron and the transferrin saturation index (TSI). Transferrin was measured by the nephelometry method in a Siemens model BNII. Ferritin levels were measured using the Abbott Access model chemiluminescence method. Serum iron was measured by dry chemistry methodology in the Johnson & Johnson® Fusion model and the TSI was calculated (21). Hemoglobin (Hb), mean corpuscular volume (MCV), corpuscular Hb concentration (CHCM) and red cell distribution width (RDW) were also determined.
Subjects whose biochemical and/or ultrasound examinations had criteria for diagnostic confirmation or staging of steatosis were submitted to ultrasound guided liver biopsy using a 16 G needle. Liver samples of 2–3 cm in length were fixed in 10% buffered formalin solution for 24 hours and processed for histological sections. The slides were stained by hematoxylin-eosin for conventional histological analysis, by Masson’s trichrome to assess the degree of fibrosis and by the histochemical method of Prussian Blue (Perls) to investigate the presence of iron deposits in liver biopsies.
Histological analysis was performed by an experienced pathologist and reviewed by a second pathologist, based on Kleiner’s criteria (17). The analysis consisted of the evaluation of: steatosis (grade I, 5–33%; grade II, 33–66%; and grade III, ˃66% of the biopsy), hepatocellular ballooning
Statistical analysis
A descriptive analysis was performed for anthropometric, clinical and laboratory variables. For the categorical variables, the simple and relative frequencies were calculated. The associations between categorical independent variables and dependent variables were assessed by comparing proportions using the Chi-square test or the Fisher’s Exact Test. A logistic regression model was used to evaluate the associations between risk factors and the presence or absence of NASH histological lesions. For logistic regression model, the results are given as odds ratios (ORs) with 95% confidence intervals (CIs). The dependent variables were: NASH histological lesions (17,22), hepatic steatosis, fibrosis, lobular and portal inflammation, hepatocellular ballooning and presence of hemosiderin deposits. The independent variables were gender, age, body weight, height, BMI, degree of obesity, WC, T2DM, MetS, arterial hypertension (AH), dyslipidemia, albumin, total bilirubin, AST, ALT, AST/ALT, GGT, ALP, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, fasting glycemia, basal insulin, HOMA-IR, Hb, mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC), platelets, RDW, ferritin, serum iron, transferrin and TSI. The level of significance was 5%.
Results
Subjects characteristics
Eighty-eight subjects, who met the inclusion criteria, were enrolled in this study. The mean age was 51.3 years old (minimum age: 21 years; and maximum age: 80 years) and the majority of the individuals according to the age group was ≥50 years old (63.6%). The highest proportion of subjects was female (80.7%) when compared to male (19.3%). Regarding the nutritional status, 49/88 subjects (55.7%) had grade 1 obesity and 39/88 (44.3%) had grades 2 or 3 obesity. The mean BMI was 35.2 kg/m2. AH was observed in 58/88 subjects (65.9%), WC, measured in 73/88 individuals, was increased in 72/73 individuals (98.6%). Of these 73 individuals, 58 (79.5%) met other criteria for MetS.
Laboratory data of subjects
T2DM, previously diagnosed or glycemia above 100 mg/dL was present in 57/88 subjects (64.8%) and TG levels above 150 mg/dL was present in 54/88 (61.4%). HOMA-IR value was increased in 66/88 individuals (75%) and basal insulin value was increased in 11/88 individuals (12.5%). HDL cholesterol levels were decreased in 62/88 subjects (70.5%) and 43/88 (48.9%) presented total cholesterol values above 200 mg/dL. Dyslipidemia was present in 75/88 individuals (85.2%). AST levels were elevated in 33/88 subjects (37.5%) and 29 (33.0%) presented high ALT values. The AST/ALT ratio was ≤1 in 66/88 subjects (75%). ALP values were elevated in 11/88 subjects (12.5%) and total bilirubin levels were elevated in 3/88 subjects (3.4%). GGT values were found above normal in 49/88 subjects (55.7%). Isolated values of GGT were found in 16/88 subjects (18.2%), most of them associated with the use of more than 4 medications. Albumin serum levels were normal in 86/88 subjects (97.7%). Serum iron mean values were 103.5 g/dL and transferrin 2.7 g/L. The mean TSI was 32.2% and the mean values of ferritin were 234 ng/mL (normal reference level 204 ng/mL). Ferritin levels were elevated in 31/88 subjects (35.2%) and 12/88 subjects (13.6%) had TSI above 45%. The mean values of Hb (14.1 g/dL), MCV (88.4 mm3), MCHC (33.3 g/dL) and RDW (13.4%) were within normal range.
Histological analysis
The assessment of hepatic steatosis showed grade 1 steatosis in 30/88 biopsies (34.1%), grade 2 in 29/88 biopsies (33.0%) and grade 3 in 29/88 biopsies (33.0%). Ballooning of hepatocytes was observed in all liver biopsies, and 83/88 biopsies (94.3%) had few ballooned cells. Fibrosis was found in 48/88 biopsies (54.5%). From the liver biopsies with fibrosis, 21/48 (43.8%) were mild and 27/48 (56.3%) had moderate/severe fibrosis. Lobular inflammation was observed in 79/88 biopsies (89.8%), portal inflammation was detected in 55/88 biopsies (62.5%) and mixed inflammation was found in 54/88 biopsies (61.4%). Analysis of stainable iron in liver biopsies by Perls method, demonstrated fine granular iron deposits in the cytoplasm of hepatocytes in 17/88 biopsies (19.3%) (Figure 1).
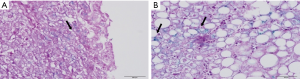
Association between hepatic histological lesions and studied variables
The relationship among NASH histological lesions: iron stain positive, steatosis, hepatocellular ballooning, fibrosis, inflammation (lobular, portal or mixed) and laboratory tests: ferritin, transferrin, serum iron, TSI, dyslipidemia, LDL, T2DM, AST/ALT, AST, ALT, ALP and GGT is shown on Table 1.
Table 1
| Nash histological lesions | Variable | OR | 95% CI | P value |
|---|---|---|---|---|
| Iron stain positive | Ferritin | 0.19 | 0.05–0.64 | 0.01* |
| Transferrin | 0.06 | 0.04–7.05 | 0.65 | |
| Serum iron | 1.36 | 0.12–15.99 | 0.8 | |
| Dyslipidemia | 0.62 | 0.11–3.41 | 0.58 | |
| Steatosis | LDL | 2.73 | 1.04–7.16 | 0.04* |
| AST/ALT | 2.94 | 1.12–7.74 | 0.02* | |
| Iron stain positive | 0.36 | 0.12–1.05 | 0.06 | |
| Hepatocellular ballooning | Dyslipidemia | 0.07 | 0.01–0.97 | 0.04* |
| AST | 12.11 | 0.91–160.67 | 0.06 | |
| Fibrosis | T2DM | 0.19 | 0.07–0.49 | 0.01* |
| Dyslipidemia | 6.49 | 1.68–25.00 | 0.01* | |
| Portal inflammation | ALP | 5.43 | 1.15–25.58 | 0.03* |
| GGT | 1.33 | 0.45–3.91 | 0.6 | |
| Iron stain positive | 0.79 | 0.18–3.44 | 0.76 | |
| Dyslipidemia | 0.25 | 0.06–1.07 | 0.06 | |
| Lobular inflammation | ALT | 4.07 | 0.79–20.74 | 0.09 |
| T2DM | 0.29 | 0.06–1.45 | 0.13 | |
| Iron stain positive | 0.79 | 0.18–3.44 | 0.96 | |
| Mixed inflammation | Dyslipidemia | 0.16 | 0.03–0.77 | 0.02* |
| ALP | 6.14 | 1.31–28.71 | 0.02* | |
| ALT | 1.06 | 0.37–3.02 | 0.91 | |
| T2DM | 0.5 | 0.18–1.40 | 0.19 | |
| Iron stain positive | 0.53 | 0.15–1.91 | 0.33 |
*, P˂0.05. OR, odds ratio; CI, confidence interval; HDL, high density lipoprotein; LDL, low density lipoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; T2DM, type 2 diabetes mellitus; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; NASH, nonalcoholic steatohepatitis.
The presence of stainable hepatic iron was not associated with NASH histological lesions. Serum ferritin and tissue iron stain had a significant negative correlation (OR 0.19; 95% CI, 0.05–0.64; P<0,01). There was a significant association between more advanced hepatic steatosis and increased values of LDL and AST/ALT. There was a significant relationship between dyslipidemia and progression of hepatic fibrosis for more advanced stages, but T2DM had a negative correlation with hepatic fibrosis. No relationship among dyslipidemia and hepatic ballooning and mixed inflammation (lobular and portal) was detected. Association between T2DM and mixed inflammation and more advanced degrees of hepatic fibrosis was not found. The presence of portal inflammation and mixed inflammation were associated with higher mean alkaline phosphatase values. Lobular inflammation, generally more prominent than portal inflammation in NASH, was not independently associated with ALT abnormal values, T2DM and iron stain positive in liver biopsies.
Discussion
This study evaluated the potential association among laboratory markers of iron metabolism, iron stain positivity in liver biopsies and the severity of NASH in a cohort of adult obese individuals. It was a prospective study that enrolled 88 subjects with liver biopsy-proven NASH. We did not find significant association between the presence of iron in the hepatic parenchyma and the intensity of NASH in this cohort of obese subjects.
Iron deficiency and anemia are frequent findings in subjects with advanced stages of obesity (23). In obesity, increased proinflammatory cytokines may stimulate hepcidin production by the liver and adipose tissue (24,25). Elevated hepcidin concentrations may explain lower duodenal ferroportin expression and diminished dietary iron absorption with decrease in serum iron (26).
Increased iron demand, owing to increased fat mass (27) or diminished iron uptake, could explain the association between obesity and iron deficiency (28). We did not find anemia in our obese subjects. This could be attributed to the characteristics of the population studied, which included mainly postmenopausal women and some insulin-resistant men (29).
Some studies have shown that increased serum ferritin is the most relevant independent predictor of histologic severity and advanced hepatic fibrosis in patients with NAFLD (30,31). We did not find statistically significant association among increased levels of serum iron, transferrin and TSI and more advanced degrees of hepatic fibrosis. Increased ferritin levels were reported to identify risk for NASH among patients with normal TSI (32). Increased ferritin levels have been observed in type II diabetes mellitus, where it is associated with increased steatosis and inflammation (33). The MetS is highly prevalent among patients with NAFLD, and particularly among patients with NASH, and metabolic disease might be amplified by iron accumulation (31,34). In our study, iron metabolism biomarkers were not related to insulin resistance parameters or other features of MetS. Increased ferritin levels in NAFLD may be an expression of a metabolic derangement and/or of hepatic damage due to widespread activation of inflammatory cytokines (35). It is likely that the metabolic derangement in our subjects with NASH had a greater effect on ferritin serum levels than on hepatic iron stores, since we have found an inverse association between increased ferritin levels and iron stain positive in liver biopsies in our cohort of obese subjects with NASH. This finding highlights that obesity and NASH, as chronic inflammatory conditions, may be associated to increased levels of ferritin, without repercussion on iron deposits in the liver. For the histological analysis of iron, we used the Perls method, which is an easy, cheap and widely used method for identification of iron deposits (36). We found Perls positivity in 17/88 biopsies (19.3%) and from these, 6 had moderate/severe fibrosis, 5 had mild fibrosis and 6 had no fibrosis. The presence of hepatic iron has been considered important for cellular oxidative stress and progression of hepatic fibrosis in NAFLD (37). In a model linking iron, cholesterol, and insulin resistance in the development of NAFLD, over-nutrition leads to insulin resistance, increased lipid deposition in the liver, and increased flux of free fatty acids from adipose tissue to the liver (38). Associated inflammation leads to increased production of leptin, which in turn increases hepatic hepcidin production, thereby increasing intracellular iron (39). This increase in intracellular iron is associated with increased hepatic cholesterol synthesis, further increasing the lipid burden to the liver. The combination of steatosis and cellular iron loading together with the increased free fatty acids, could exacerbate the progression from steatosis to NASH and its hepatic complications (40). Manco et al. [2011], in a study on the relationship between hepatic iron content and insulin resistance in children with biopsy-proven NAFLD, have found low hepatic iron deposition in 22% of subjects, similar to our findings, without association with HFE mutations (41).
In patients with untreated NAFLD, increased portal inflammation has been proposed as a marker of severity of liver disease (42). We didn’t observe association between portal inflammation and more advanced stages of hepatic fibrosis. Moreover, our study did not show association between diabetes and advanced hepatic fibrosis. This might be attributed to the use of certain medications, like statins, that can reduce the progression of hepatic fibrosis in patients with NAFLD (43-45).
In the present study, the histological evaluation of hepatic iron did not show significant association with the degree of fibrosis or steatosis. Moreover, no relationship between hepatic iron deposition and more advanced clinical outcomes was found. We identified fine iron granular deposits in the cytoplasm of hepatocytes. This finding does not appear to characterize iron overload in the hepatic parenchyma. The presence of iron within hepatocytes could be interpreted as iron in transit, a physiological event related to iron metabolism in the hepatic cell. Zamin et al. [2006] did not establish a primary role for iron in the pathogenesis of NASH, since they did not find association among iron deposits, fibrosis and inflammatory activity in the liver (46). Chandok et al. [2012] demonstrated that hyperferritinemia is common among patients with NAFLD, but increased serum ferritin does not correlate with advanced stages of fatty liver disease (47). Therefore, it does not appear that iron has an important role in the pathophysiology of NASH, as well as on the different stages of hepatic fibrosis. In conclusion, the findings from our study did not confirm the association between the presence of iron in the hepatic parenchyma and the intensity of NASH in obese subjects.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.04.01). Fernando Gomes Romeiro serves as an unpaid editorial board member of AME Medical Journal from Apr 2017 to Apr 2019. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local Hospital Ethical Committee (No. 3999-2011). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol 2005;43:508-14. [Crossref] [PubMed]
- Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2010;3:121-37. [Crossref] [PubMed]
- Ferrer Márquez M, Rico Morales Mdel M, Carvia Pousaille C, et al. Prevalence and associated factors to non-alcoholic steatohepatitis in obese patients subjected to bariatric surgery. Cir Esp 2008;84:313-7. [PubMed]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85. [Crossref] [PubMed]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592-609. [Crossref] [PubMed]
- Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010;5:145-71. [Crossref] [PubMed]
- Wilkins T, Tadkod A, Hepburn I, et al. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician 2013;88:35-42. [PubMed]
- Hu KC, Wang HY, Liu SC, et al. Nonalcoholic fatty liver disease: updates in noninvasive diagnosis and correlation with cardiovascular disease. World journal of gastroenterology World J Gastroenterol 2014;20:7718-29. [Crossref] [PubMed]
- Sanyal AJAmerican Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002;123:1705-25. [Crossref] [PubMed]
- Actis GC, Olivero A, Lagget M, et al. The practice of percutaneous liver biopsy in a gastrohepatology day hospital: a retrospective study on 835 biopsies. Dig Dis Sci 2007;52:2576-9. [Crossref] [PubMed]
- Dever J, Kowdley KV. Iron metabolism and diagnosis of iron overload disorders. Expert Opin Med Diagn 2010;4:67-77. [Crossref] [PubMed]
- Fargion S, Valenti L, Fracanzani AL. Beyond hereditary hemochromatosis: new insights into the relationship between iron overload and chronic liver diseases. Dig Liver Dis 2011;43:89-95. [Crossref] [PubMed]
- Pietrangelo A. Iron in NASH, chronic liver diseases and HCC: how much iron is too much? J Hepatol 2009;50:249-51. [Crossref] [PubMed]
- Fujita N, Takei Y. Iron overload in nonalcoholic steatohepatitis. Adv Clin Chem 2011;55:105-32. [Crossref] [PubMed]
- Pietrangelo A. Metals, oxidative stress, and hepatic fibrogenesis. Semin Liver Dis 1996;16:13-30. [Crossref] [PubMed]
- Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1-452. [PubMed]
- Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313-21. [Crossref] [PubMed]
- Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999;31:929-38. [Crossref] [PubMed]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421. [PubMed]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9. [Crossref] [PubMed]
- Kalantar-Zadeh K, Hoffken B, Wunsch H, et al. Diagnosis of iron deficiency anemia in renal failure patients during the post-erythropoietin era. Am J Kidney Dis 1995;26:292-9. [Crossref] [PubMed]
- Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-74. [Crossref] [PubMed]
- Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014;6:3587-600. [Crossref] [PubMed]
- Bekri S, Gual P, Anty R, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788-96. [Crossref] [PubMed]
- Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271-6. [Crossref] [PubMed]
- Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-3. [Crossref] [PubMed]
- Nead KG, Halterman JS, Kaczorowski JM, et al. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114:104-8. [Crossref] [PubMed]
- Pinhas-Hamiel O, Newfield RS, Koren I, et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord 2003;27:416-8. [Crossref] [PubMed]
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, et al. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity. 2010;18:1449-56. [Crossref] [PubMed]
- Kowdley KV, Belt P, Wilson LA, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012;55:77-85. [Crossref] [PubMed]
- Bugianesi E, Manzini P, D'Antico S, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology 2004;39:179-87. [Crossref] [PubMed]
- Fargion S, Mattioli M, Fracanzani AL, et al. Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol 2001;96:2448-55. [Crossref] [PubMed]
- Turlin B, Mendler MH, Moirand R, et al. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol 2001;116:263-70. [Crossref] [PubMed]
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917-23. [Crossref] [PubMed]
- Sibille JC, Kondo H, Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology 1988;8:296-301. [Crossref] [PubMed]
- Turlin B, Deugnier Y. Evaluation and interpretation of iron in the liver. Semin Diagn Pathol 1998;15:237-45. [PubMed]
- Dongiovanni P, Fracanzani AL, Fargion S, et al. Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol 2011;55:920-32. [Crossref] [PubMed]
- Sharp PA. New insights into the role of iron in the development of nonalcoholic fatty liver disease. Hepatology 2010;52:408-10. [Crossref] [PubMed]
- Barisani D, Pelucchi S, Mariani R, et al. Hepcidin and iron-related gene expression in subjects with Dysmetabolic Hepatic Iron Overload. J Hepatol 2008;49:123-33. [Crossref] [PubMed]
- George DK, Goldwurm S, MacDonald GA, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology 1998;114:311-8. [Crossref] [PubMed]
- Manco M, Alisi A, Fernandez Real JM, et al. Early interplay of intra-hepatic iron and insulin resistance in children with non-alcoholic fatty liver disease. J Hepatol 2011;55:647-53. [Crossref] [PubMed]
- Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-Clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009;49:809-20. [Crossref] [PubMed]
- Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1224-9, 1229.e1-2.
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. [Crossref] [PubMed]
- Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943-51. [Crossref] [PubMed]
- Zamin I Jr, Mattos AA, Mattos AZ, et al. Prevalence of the hemochromatosis gene mutation in patients with nonalcoholic steatohepatitis and correlation with degree of liver fibrosis. Arq Gastroenterol 2006;43:224-8. [Crossref] [PubMed]
- Chandok N, Minuk G, Wengiel M, et al. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis 2012;21:53-8. [PubMed]
Cite this article as: da Silva MP, Caramori CA, Kurokawa CS, Corrente JE, Romeiro FG, Rodrigues MAM. Relationship among biomarkers of iron metabolism and severity of underlying nonalcoholic steatohepatitis (NASH). AME Med J 2018;3:58.

