Prognostic significance of venous tumor thrombus consistency (solid vs. friable) in patients with renal cell carcinoma: A systematic review and meta-analysis
Introduction
Renal cell carcinoma (RCC) represents 2–3% of all cancers, with an estimated 63,990 new cases and 14,400 deaths in 2017 in the United States (1). In the newly diagnosed cases, the number of patients with RCC forming a venous tumor thrombus (VTT) and invading the inferior vena cava (IVC) occupied 4–10% (2). RCC tumor thrombus could extend to the renal vein (RV), the IVC, or even the heart, and an RCC tumor thrombus in the IVC was a significant adverse predictive of survival in patients with RCC (3).
Radical nephrectomy with thrombectomy might have revealed an improved prognosis and a better effect of subsequent targeted therapy than other treatments and become a preferred treatment option (4,5). Several different surgery techniques of thrombectomy were applied to the patients, according to different thrombus levels, including open surgery, pure laparoscopy, robotic-assisted laparoscopy (6). However, each kind of surgery can be most appropriate or efficacious only in a subset of patients (7). Therefore, a more precise prognostic prediction might be useful for surgical selection.
Recently, venous tumor thrombus consistency (VTTC) was defined as solid or friable from the morphologic aspect of the tumor thrombus (8). Typically, Bertini et al. suggested that the existence of a friable thrombus was an independent prognostic factor of cancer-specific survival (CSS) in the patients with RCC (8). To date, previous studies have investigated the effect of VTTC on the survival of RCC (7,8). However, controversies exist about the effect to the prognosis of RCC. In addition, several prognostic clinicopathological features including TNM classification, Fuhrman grading system, RCC subtypes, microvascular invasion, tumor necrosis and invasion of the collecting system have been considered as an independent predictive factor of RCC (3). Nevertheless, the definite prognostic significance of VTTC in patients with patients with RCC is still unclear (9-12).
Until now, there were five articles discussing about the effect of VTTC on the prognosis of patients with RCC, but the available results remained contradictory (7,8,13-15). Hence, we conducted a meta-analysis including all eligible case-control studies for the first time to investigate whether VTTC was an independent prediction of the patients with RCC.
Methods
Search strategy
This meta-analysis was conducted according to the guideline of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (16). No ethical approval was required due to the fact that all included articles have been published. We searched PubMed, EMBASE, Web of Science and the Cochrane Library electronic databases comprehensively to evaluate the prognostic role of VTTC in patients with RCC, up to December 1st, 2017. The key search words included “tumor thrombus consistency” or “neoplasms thrombosis consistency” “prognosis” or “survival”, and “renal cancer” or “renal cell carcinoma” or “kidney cancer”. In addition to electronic search original papers, references of relevant articles were manually retrieved for potential eligible trials. Moreover, no language limitation existed in this search process.
Selection criteria
Studies were included in this meta-analysis only if they met the following inclusion criteria: (I) RCC patients with VTT confirmed by histopathological examination and underwent nephrectomy with thrombectomy; (II) studies analyzing the prognostic role of VTTC in patients with RCC; (III) outcomes of studies included overall survival (OS) or cancer-special survival (CSS); (IV) sufficient data from the included studies could be extracted. Accordingly, the exclusive criteria were as follows: (I) case reports or editorials or review articles; (II) studies not related to RCC or VTTC; (III) unavailable information or complete data; (IV) duplicates of previous publication.
Data extraction
Two independent investigators assessed the titles and abstracts of all included articles and guaranteed the eligible studies for next analysis. Data from the included studies were independently extracted with a unified items form. Additionally, any disagreement or uncertainty was brought to a group discussion until it came to a consensus. The following information were recorded: first author’ name, publication year, country, sample size, the number of cases with solid VTT and friable VTT respectively, median of patient age, follow-up and survival data including OS or CSS.
Quality assessment
Newcastle Ottawa Scale (NOS) was used to evaluate the quality of the studies, including case-control and cohort studies (17). A study could be awarded a maximum of one star for each point within the selection and exposure categories, and a maximum of two stars can be given for comparability. We considered studies with scores of more than 7 as high-quality studies and only high-quality studies were included in our meta- analysis.
Statistical analysis
The pooled hazard ratio (HR) and its 95% confidence intervals (CIs) were calculated to assess the prognostic impact of VTTC on RCC patients. Besides, the associations between VTTC and clinical parameters of RCC were calculated using the pooled odds ratio (OR) with 95% CIs. Cochrane Q test and Higgins I2 statistic were used to evaluate the statistical heterogeneity among included studies. If the heterogeneity was acceptable (I2>50% suggested obvious heterogeneity), the fixed-effect model (Mantel-Haenszel method) would be adopted. Otherwise, the random-effect model (DerSimonian-Laird method) would be applied. To test the reliability and stability of all the pooled outcomes, sensitivity analysis was performed by sequential omission of individual studies. In addition, publication bias was assessed using Begg’s funnel plots and Egger’s linear regression test. Stata software (version 12.0; StataCorp LP, College Station, TX) was utilized to dispose all above statistical analyses and a P value <0.05 was considered statistically significant.
Results
Studies characteristics
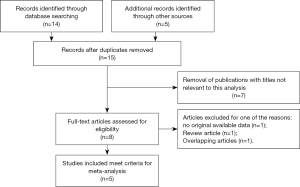
Based on the retrieve strategy above, a total of 19 relevant articles were retrieved from the aforementioned databases and other sources. Four duplicated articles were excluded using literature manager software. Then, 7 unrelated articles were excluded after screening titles and abstracts of the remaining articles carefully. Among the remaining 8 articles for full text evaluation, 1 article lacked original available data, 1 article belonged to review articles and 1 article had overlapping data. Finally, 5 articles met the inclusive criteria and were ultimately adopted in the present meta-analysis (Figure 1) (7,8,13-15). The baseline characteristics of these included studies were shown in Table 1. The 5 articles contained 1,018 cases of RCC patients with VTT, which were all diagnosed by histopathological methods. These patients were from three countries (Poland, Germany and Italy) with the follow-up more than 9 mouths. The relevant prognostic parameters of these included articles contained OS or CSS. Moreover, NOS scores assessing the study quality ranged from 7 to 9, which were considered adequate for the following meta-analysis.
Table 1
| Author | Year | Country | No of cases | No of cases with friable VTTC | No of cases with solid VTTC | Median (mean) age | Follow-up (months) | Outcome | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Golabek et al. (14) | 2016 | Poland | 84 | 46 | 38 | 64.5 (26.0–84.0) | 9 [1–66] | OS | 7 |
| Mager et al. (15) | 2016 | Germany | 413 | 188 | 225 | 61.5 (20.0–84.0) | 50 [0–328] | CSS | 8 |
| Antonelli et al. (7) | 2015 | Italy | 147 | 68 | 79 | 66.0 (35.5–85.0) | 40.5 [1–215] | OS, CSS | 8 |
| Weiss et al. (13) | 2013 | Germany | 200 | 20 | 130 | 65.8 (37.0–86.0) | 49 | OS | 7 |
| Bertini et al. (8) | 2011 | Italy | 174 | 67 | 107 | 62.5 (26.0–83.0) | 24 [2–220] | CSS | 9 |
VTCC, venous tumor thrombus consistency; OS, overall survival; CSS, cancer-specific survival; NOS, Newcastle-Ottawa Scale.
Quantitative synthesis results
Prognostic role of VTTC in patients with RCC
Friable VTT was an important adverse predictor of OS in patients with RCC (pooled HR =1.60; 95% CI, 1.13–2.26) (Figure 2A). There was no prominent heterogeneity (P=0.514) in eligible studies, and the pooled HR for OS was performed using the fixed-effort model. Nevertheless, the results showed that friable VTT was not a significant predictor of CSS in patients with RCC (pooled HR =1.32; 95% CI, 0.88–1.99) (Figure 2B).

Association of RCC clinicopathological variables between friable VTT and solid VTT
To further investigate the association of RCC clinicopathological variables between friable VTT and solid VTT, we selected some confirmed clinicopathological parameters as independent predictive factors of RCC, such as distant metastases, nodal status, perinephric fat invasion, Fuhrman grade, pathologic stage, histological subtype and so on. The results about the associations on clinicopathologic features between Friable VTTC and Solid VTTC with RCC were shown in Table 2. Of the selected variables, nodal status (pN+ vs. pN0/cN0) (OR =1.48; 95% CI, 1.10–2.01; P=0.214), perinephric fat invasion (yes vs. no) (OR =1.49; 95% CI, 1.14–1.97; P=0.301), tumor necrosis (yes vs. no; OR =2.75; 95% CI, 1.88–4.01; P=0.135), Fuhrman grade (≥3 vs. <3; OR =1.58; 95% CI, 1.12–2.21; P=0.153), pathologic grade (>3 vs. ≤3; OR =3.19; 95% CI, 1.92–5.29; P=0.393) and histological subtype (clear cell RCC vs. others; OR =2.71; 95% CI, 1.01–7.26; P=0.015) were associated with VTTC. However, outcomes showed that there were no significantly differences between friable VTT and solid VTT in clinicopathological variables of RCC, including distant metastases, sarcomatoid differentiation, venous wall invasion and pathologic stage.
Table 2
| Variables | No. of studies | No. of cases with Friable VTTC | No of cases with solid VTTC | Effects model | OR (95% CI)* | P& | I-squared (%) |
|---|---|---|---|---|---|---|---|
| Distant metastases (yes vs. no) | 5 | 389 | 579 | Random | 1.25 (0.95–1.65) | 0.010 | 69.6 |
| Nodal status (pN+ vs. pN0/cNo) | 5 | 389 | 579 | Fixed | 1.48 (1.10–2.01) | 0.214 | 31.1 |
| Perinephric fat invasion (yes vs. no) | 5 | 389 | 579 | Fixed | 1.49 (1.14–1.97) | 0.301 | 17.9 |
| Sarcomatoid differentiation (yes vs. no) | 4 | 201 | 354 | Fixed | 0.91 (0.50–1.67) | 0.795 | 0.0 |
| Tumour necrosis (yes vs. no) | 4 | 201 | 354 | Fixed | 2.75 (1.88–4.01) | 0.135 | 46.0 |
| Venous wall invasion (yes vs. no) | 2 | 234 | 263 | Fixed | 1.23 (0.84–1.80) | 0.444 | 0.0 |
| Fuhrman grade (≥3 vs. < 3) | 3 | 254 | 393 | Fixed | 1.58 (1.12–2.21) | 0.153 | 46.7 |
| Pathologic stage (>T3 vs. ≤T3) | 4 | 321 | 500 | Fixed | 1.61 (0.97–2.68) | 0.164 | 41.3 |
| Pathologic grade (>3 vs. ≤3) | 2 | 135 | 186 | Fixed | 3.19 (1.92–5.29) | 0.393 | 0.0 |
| Histological subtype (clear cell RCC vs. others) | 4 | 322 | 472 | Random | 2.71 (1.01–7.26) | 0.015 | 71.4 |
*, random-effects model was used when P value for heterogeneity test <0.1; otherwise, fixed-effects model was used. &, P value of Q test for heterogeneity. VTCC, venous tumor thrombus consistency; OR, odds ratio; CI, confidence interval; RCC, renal cell carcinoma.
Sensitivity analysis
Sensitivity analysis was utilized to detect the influence of each study on the pooled HR by repeating the meta-analysis, while omitting one single study each time. The sensitivity analysis for friable VTT and solid VTT in the patients with RCC was shown in Figure 3, demonstrating that no individual study significantly affected the pooled HR. Thus, sensitivity analysis showed that our results were reliable.

Publication bias
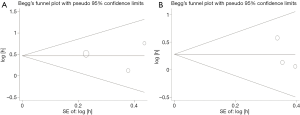
The Begg’s funnel plot was applied to assess the publication bias of the literature, and the shapes of them seemed no evidence of obviously asymmetrical, indicating no significant publication bias, which was also confirmed according to funnel plot (OS: Begg’s test, P=0.602; Egger’s test, P=0.604. CSS: Begg’s test, P=0.117; Egger’s test, P=0.383; Figure 4). Therefore, the overall outcomes indicated that our results were statistically robust.
Discussion
Advanced RCC with VTT is considered as a grave disease with an unfavorable prognosis and the treatment of this disease remains challenging and controversial (3). Several series articles reported a 5-year survival rate ranging from 25% to 57% (11,18,19). Complete surgical resection was still regarded as the most effective and standard therapeutic approach in general because of the barely satisfactory effect of targeted therapy on the treatment of RCC (4,5,20-22). In addition, the classification method for VTT was not abundant and only Mayo classification was accepted widely (15). Recently, Bertini et al. tried to standardise the pathologic definition of VTTC as a solid thrombus or a friable thrombus and reported the presence of a friable thrombus significantly affected patient prognosis (8). Since then, the prognostic significance of VTTC (solid vs. friable) in patients with RCC has attracted extensive attention and several studies have been conducted to explore it (8,13,14). However, the results were still unclear and even controversial. Therefore, we conducted a comprehensive meta- analysis to clarify the prognostic value of VTTC in patients with RCC for the first time. Meanwhile, we also further investigated the association between RCC clinicopathological variables and VTTC.
Meta-analysis, as a powerful tool, could provide more reliable results than a single study especially in explaining controversial conclusions. As a consequence, we took advantage of meta-analysis to clarify the accurate results about the prognostic value of VTTC in RCC. This meta-analysis demonstrated friable VTT was an adverse predictor of OS in patients with RCC, which was consistent with the result reported by Weiss et al. (13) However, in subsequent analysis, our results indicated that friable VTT was not a significant predictor of CSS in patients with RCC, which were inconsistent with the study conducted by Bertini et al. (8) The possible reasons about such different results were as follows. On the one hand, marked differences in the number of patients with friable VTT versus solid VTT were observed among the studies. The various proportions of VTTC might interfere with the potential correct results. On the other hand, inhomogeneous distribution of clinicopathologic features might have an effect on the patients’ survival, such as nodal status, perinephric fat invasion, tumor necrosis, Fuhrman grade, histological subtype and so on (3). Up to now, the impact of VTTC on the prognosis of RCC patients was still uncertain. Therefore, more high-quality and multicentric studies with larger sample sizes were needed to investigate the the association between VTTC and the survival of RCC patients.
In this meta-analysis, we used both OS and CSS as the endpoint of our study. As the cause of death could not have been determined reliably in all patients, CSS would have been likely affected and thus OS was a more robust measure than CSS. Hence, it should be noted that OS was the gold standard for cancer clinical research and was considered as the most preferred and reliable cancer endpoint according to the US Food and Drug Administration, which should be used in preference to CSS (23). Thus, the findings of our current meta-analysis suggested that the presence of a friable VTT might significantly affect the prognosis of the patients with RCC.
With regard to the clinicopathological features, we found that nodal status, perinephric fat invasion, tumor necrosis, Fuhrman grade, pathologic grade and histological subtype were associated with VTTC in the patients with RCC (3,24). Our results showed that patients with friable VTT were correlated with nodal status (pN+), perinephric fat invasion and tumor necrosis rather than distant metastases. One possible reason for these discrepancies was that the studies included in the meta-analysis about distant metastases existed significant heterogeneity (P=0.010, I2=69.6%), which might have a pronounced effect on the results. Interestingly, as for histological subtype, we found that a friable VTT was most commonly associated with a clear cell histological subtype. However, Weiss et al. suggested that papillary RCC was more frequent in patients with friable VTT than these with solid VTT (13). Furthermore, we showed that friable VTT was significantly associated with a higher pathologic grade. With regard to Fuhrman grade, patients with friable VTT were correlated with Fuhrman grade III or IV. Similarly, Martínez-Salamanca et al. reported that Fuhrman grade is an independent predictor of CSS in both univariate and multivariate analysis and Fuhrman grade III or IV were the strongest predictors of worse survival (25). The latest version of the TNM classification was published in 2010 and has been proved to be the most reliable prognostic factor in both single- and multi-institutional studies (3,26). Since lacking data about tumor classification, we could not study it in depth. Thus, further studies needed to clarify this point. In this study, our analysis indicated that the clinicopathological variables of RCC were closely associated with VTTC (solid vs. friable).
To a certain extent, several limitations in our meta-analysis should be taken into consideration when interpreting the data. Firstly, most of the included articles were retrospective studies, and the relatively small sample size and short-term follow-up also rendered the power of our conclusion less reliable, which might be subject to the bias and limitations inherent to this type of study. Thus, the data used in the analyses should be also obtained from a prospectively maintained database, which reduced the risk of errors and/or omissions. Secondly, only 5 studies were adopted in this meta-analysis, which might inevitably increase the risk of random error. Therefore, more high-quality and multicentric studies were required to explore the prognostic role of VTTC in patients with RCC. Thirdly, most of studies included could have been influenced by involvement of multiple surgeons and different therapies after disease recurrence, which might contribute to biases of this meta-analysis. In addition, the additional targeted therapy of patients with RCC during the study period might also lead to a bias when analysing survival data.
Conclusions
The results of the present meta-analysis indicated that the presence of a friable VTT could be an adverse prognostic factor of patients with RCC. Taking into account the limited the number of articles, more high-quality and multicentric studies with larger sample sizes were needed to investigate the association between VTTC and the prognosis of RCC patients.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.05.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No ethical approval was required due to the fact that all included articles have been published.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Lardas M, Stewart F, Scrimgeour D, et al. Systematic Review of Surgical Management of Nonmetastatic Renal Cell Carcinoma with Vena Caval Thrombus. Eur Urol 2016;70:265-80. [Crossref] [PubMed]
- Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913-24. [Crossref] [PubMed]
- Haferkamp A, Bastian PJ, Jakobi H, et al. Renal cell carcinoma with tumor thrombus extension into the vena cava: prospective long-term followup J Urol 2007;177:1703-8. [Crossref] [PubMed]
- Kirkali Z, Van Poppel H. A critical analysis of surgery for kidney cancer with vena cava invasion. Eur Urol 2007;52:658-62. [Crossref] [PubMed]
- Shao P, Li J, Qin C, Lv Q, et al. Laparoscopic Radical Nephrectomy and Inferior Vena Cava Thrombectomy in the Treatment of Renal Cell Carcinoma. Eur Urol 2015;68:115-22. [Crossref] [PubMed]
- Antonelli A, Sodano M, Sandri M, et al. Venous tumor thrombus consistency is not predictive of survival in patients with renal cell carcinoma: A retrospective study of 147 patients. Int J Urol 2015;22:534-9. [Crossref] [PubMed]
- Bertini R, Roscigno M, Freschi M, et al. Impact of venous tumour thrombus consistency (solid vs friable) on cancer-specific survival in patients with renal cell carcinoma. Eur Urol 2011;60:358-65. [Crossref] [PubMed]
- Kuroda N, Karashima T, Inoue K, et al. Review of renal cell carcinoma with rhabdoid features with focus on clinical and pathobiological aspects. Pol J Pathol 2015;66:3-8. [Crossref] [PubMed]
- Al Otaibi M, Abou YT, Alkhaldi A, et al. Renal cell carcinoma with inferior vena caval extention: impact of tumour extent on surgical outcome. BJU Int 2009;104:1467-70. [Crossref] [PubMed]
- Wagner B, Patard JJ, Mejean A, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol 2009;55:452-9. [Crossref] [PubMed]
- Bertini R, Roscigno M, Freschi M, et al. The extent of tumour fat invasion affects survival in patients with renal cell carcinoma and venous tumour thrombosis. BJU Int 2011;108:820-4. [PubMed]
- Weiss VL, Braun M, Perner S, et al. Prognostic significance of venous tumour thrombus consistency in patients with renal cell carcinoma (RCC). BJU Int 2014;113:209-17. [Crossref] [PubMed]
- Gołąbek T, Przydacz M, Okon K, et al. Tumour thrombus consistency has no impact on survival in patients with renal cell carcinoma. Pol J Pathol 2016;67:145-50. [Crossref] [PubMed]
- Mager R, Daneshmand S, Evans CP, et al. Renal cell carcinoma with inferior vena cava involvement: Prognostic effect of tumor thrombus consistency on cancer specific survival. J Surg Oncol 2016;114:764-8. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Cota GF, de Sousa MR, Fereguetti TO, et al. Efficacy of anti-leishmania therapy in visceral leishmaniasis among HIV infected patients: a systematic review with indirect comparison. PLoS Negl Trop Dis 2013;7:e2195 [Crossref] [PubMed]
- Pouliot F, Shuch B, Larochelle JC, et al. Contemporary management of renal tumors with venous tumor thrombus. J Urol 2010;184:833-841, 1235. [Crossref] [PubMed]
- Glazer AA, Novick AC. Long-term followup after surgical treatment for renal cell carcinoma extending into the right atrium. J Urol 1996;155:448-50. [Crossref] [PubMed]
- Flanigan RC, Mickisch G, Sylvester R, et al. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol 2004;171:1071-6. [Crossref] [PubMed]
- Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704-710. [Crossref] [PubMed]
- Moinzadeh A, Libertino JA. Prognostic significance of tumor thrombus level in patients with renal cell carcinoma and venous tumor thrombus extension. Is all T3b the same? J Urol 2004;171:598-601. [Crossref] [PubMed]
- Food and Drug Administration, Guidance for Industry Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics, (FDA, Rockville, MD, 2007). Available online: mhttp://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/ Guidances/UCM071590.pdf, Accessed on 01.01.2016.
- Ficarra V, Galfano A, Mancini M, et al. TNM staging system for renal-cell carcinoma: current status and future perspectives. Lancet Oncol 2007;8:554-558. [Crossref] [PubMed]
- Martínez-Salamanca JI, Huang WC, Millan I, et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011;59:120-7. [Crossref] [PubMed]
- Kim SP, Alt AL, Weight CJ, et al. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. J Urol 2011;185:2035-9. [Crossref] [PubMed]
Cite this article as: Qin Z, Li R, Qin Y, Han P, Tang J, Zhang L, Wang F, Qi X, Zhang W. Prognostic significance of venous tumor thrombus consistency (solid vs. friable) in patients with renal cell carcinoma: A systematic review and meta-analysis. AME Med J 2018;3:67.