
Endobronchial ultrasound-guided-transbronchial needle injection for direct therapy of lung cancer
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in the United States (1). Despite multimodal systemic and local treatment options, including intravenous chemotherapy or immunotherapy, surgical resection, and external beam radiation therapy (EBRT), the disease commonly advances. Recurrent or progressive lung cancer tends to be even less responsive to therapy, resulting in a median overall survival of approximately 5 months (2).
In the last decade, there have been dramatic improvements in the technology available to access the lung. The most notable of these achievements is the development of the curvilinear endobronchial ultrasound (EBUS). This bronchoscope contains a 7.5 Hz linear curved-array ultrasonic transducer integrated into the bronchoscope allowing real-time visualization of structures within ~4 cm of the airway wall (3). These areas may then be sampled by placing a needle through the working channel of the EBUS to perform transbronchial needle aspiration (TBNA). The rate of use of this technology has dramatically increased over the last decade. More than 85% of pulmonary and critical care programs now have the ability to perform EBUS-TBNA (4). The increasing prevalence of EBUS-TBNA systems has led to both expanded indications for performing the procedure and novel applications of the technology.
In addition to a very high yield for diagnosis, EBUS-TBNA is commonly utilized for mediastinal staging of lung cancer. The most recent edition of the American College of Chest Physicians Guidelines for Lung Cancer recommends EBUS-TBNA as the first-line approach for invasive mediastinal staging of NSCLC for patients with enlarged or PET positive mediastinal or hilar lymph nodes, or in those with radiographically negative mediastinal lymph nodes and a centrally located tumor (5). This has led to an increasing role for bronchoscopy in the initial diagnosis and staging of lung cancer. The resultant expertise with the EBUS bronchoscope, coupled with a recognition of the desperate need for novel and effective therapies for thoracic malignancies, has led to increasing interest in leveraging this technique for therapeutic effect. This interest is bolstered by the overall safety of the procedure. A large systematic review including over 1,500 patients identified no serious complications of EBUS-TBNA and only agitation, cough, and blood at the puncture site as minor complications (6). Prospective databases report rates of complication of EBUS-TBNA of approximately 1%, although complications including mediastinitis, pericarditis, and death have been reported (7,8).
The goal of this review is to outline the current status of EBUS for treatment of lung cancers, with particular focus on endobronchial ultrasound guided transbronchial needle injection (EBUS-TBNI). Initial aspects of the article will focus on the use of EBUS-TBNI with cisplatin for locoregional recurrence in a previously irradiated field (or in cases where external beam radiation is contraindicated). Less frequently used agents, and the many unknowns related to the procedure will be then be discussed.
Current therapies for locoregional lung cancer recurrence in a radiated field
Lung cancer is staged numerically (I–IV) based on characteristics of the tumor, pattern of involvement of regional lymph nodes, and presence of metastatic disease. Stages I–III may potentially be cured with surgery, radiation therapy, or systemic chemotherapy. Chemotherapy is commonly used in stages II–IV. The initial chemotherapy regimen is generally platinum based (cisplatin or the related drug, carboplatin) and another agent. Unfortunately, many patients have tumor that recurs after chemotherapy, surgery, and curative-intent EBRT.
Locoregional recurrence is defined as tumor recurrence in the treated radiation area, at the surgical margins, or in the regional lymph nodes. Despite radiation and first-line chemotherapy with two agents as many as 60% of patients with Stage III NSCLC will develop a locoregional recurrence (9,10). Even in resected early stage disease (stage I–II) the rate of locoregional recurrence exceeds 20% (11). Among these patients the most common single cause of death is respiratory failure, occurring in 30–40% of patients with lung cancer. In the vast majority of these cases, respiratory failure is at least partially attributable to growth of the tumor in the lung (12).
Unfortunately, options for these patients are limited. Second-line chemotherapy with a single agent chemotherapy or immunotherapy is considered the standard of care for recurrent disease. However, single agent chemotherapy results in only mild improvement in progression free survival with a median overall survival that is unchanged compared to placebo and remains at approximately 5 months while immunotherapy only results in response in approximately 20% of patients. Moreover, 5–15% of patients receiving single agent chemotherapy (e.g., docetaxel) or immunotherapy with nivolumab discontinue treatment due to toxicity (2,13). Thus, current therapies for treatment of recurrence or progression in a previously radiated field have limited efficacy and are associated with significant rates of adverse events.
The limited efficacy of systemic therapies has led to considerable interest in reirradiation. Unfortunately, there are few prospectively collected or randomized series to guide decision making in the setting of lung cancer recurrence in the central regions of the lungs and, in the authors opinion, interpretation of these data is often confounded. Reirradiation (generally via a stereotactic approach) for recurrence of a peripheral lung cancer within a previously radiated field appears to have high rates of local control. Similarly, low dose palliative reirradiation (e.g., ~8 Gy) appears to be somewhat effective at improving symptoms of airway obstruction and superior vena cava syndrome (14), although other studies have demonstrated that EBRT consistently fails to control symptoms of shortness of breath in patients with advanced disease (28–51%) (15). However, the appropriate comparator is high-dose reirradiation with the goal of disease control. In the largest series of patients treated with high dose (e.g., “radical”) repeat radiotherapy, 90% of patients failed therapy with approximately half recurring locally (16). However, this study included patients that had recurrence after initial radiation in sites that were not previously treated with EBRT. Following reirradiation, the rate of local recurrence is even higher, with ~88% failing in the radiated field. Further, 17% of patients in the cohort experienced grade 3 or 4 esophageal or pulmonary toxicity. In univariate analysis, recurrence outside the original radiation field was one of the few variables associated with local control. Adjusting for known and identified confounders, demonstrated that only concomitant systemic chemotherapy was associated with improvement in local control or survival. In a separate prospective multicenter cohort of 52 patients treated with reirradiation for NSCLC recurrence, 55% developed grade 3 toxicity or higher including Grade 5 toxicity (e.g., fatal hemoptysis, tracheoesophageal fistula, sepsis, pneumonitis resulting in respiratory failure, and severe esophagitis requiring parenteral nutrition) in 12%. The strongest predictor of complications was proximity to the central airways. These data raise serious questions regarding the benefit-to-risk ratio of high dose reirradiation for centrally occurring lung cancers.
The evolving role of EBUS-TBNI for direct therapy of lung cancer
TBNI under direct visualization, without the guidance of real-time ultrasound, is well established. Cytotoxic agents, including ethanol, 5-fluorouracil, and mitomycin, as well as immunostimulatory molecules have all been delivered into malignant endobronchial lesions using TBNI with direct visualization (17). Mehta and colleagues published one of the largest series to date, treating 22 patients with TBNI (without ultrasound) using direct injection of cisplatin into endobronchial tumor (18). No short term adverse events were noted.
The general experience with TBNI under direct visualization has led to increasing interest in the use of these direct therapies (e.g., treatment of the tumor with minimal exposure of normal tissues) for treatment of lesions located outside of the airway wall. In this setting, the EBUS bronchoscope is an ideal tool to guide delivery of agents into these areas. By providing real-time ultrasound guidance the operator can more accurately deliver agents into these “extrinsic” lesions (e.g., outside the airway wall). The first application of EBUS-TBNI was reported from Turkey. These authors used EBUS-TBNI to deliver cisplatin into extrinsically located tumors as adjuvant therapy (delivered during “off” weeks) to systemic chemotherapy in 5 patients with Stage IV lung cancer who had not previously been treated. Patients were excluded if they were receiving immunotherapy or radiation therapy. A dose up to 40 mg of cisplatin was used during each of the five EBUS-TBNI procedures. These were performed in the weeks between the two intravenous cycles of dose-reduced platinum doublet chemotherapy. Although this study provides an interesting approach there are a number of important limitations. First, the majority of the patients had high-grade adverse events, including significant bleeding, vomiting, leukopenia that occurred secondary to EBUS-TBNI, esophageal infiltration of cisplatin, and mediastinitis. Second, full dose intravenous doublet chemotherapy therapy has been associated with therapeutic response in randomized trials (19). A reduction to 70% of the intravenous dose was empirically chosen for use in this study, raising the specter that full dose IV therapy would have potentially resulted in greater therapeutic effect (and/or more adverse events) in combination with EBUS-TBNI of cisplatin. This study remains difficult to interpret given that it was not designed as a traditional Phase I dose-ranging study, there were very high rates of Grade 4/5 adverse events, and that two variables were changed simultaneously: the addition of intratumoral cisplatin and the dose reduction of IV chemotherapy were both altered but not specifically evaluated (for example in a factorial trial). Thus, EBUS-TBNI cannot be currently recommended as first-line therapy for any stage of NSCLC. This includes in combination with immunotherapy, given that there is an unknown safety profile, the potential for synergistic adverse events, and a lack of evidence of efficacy.
However, there is data to support the use of EBUS-TBNI with cisplatin for treatment of locoregionally recurrent lung cancer in a previously radiated field. Our group published the first report of EBUS-TBNI of cisplatin for treatment of a locally recurrent squamous cell carcinoma following initial radiation and chemotherapy (20). However, like many ideas that have reached their “time”, other groups were already evaluating this therapy. Mehta and colleagues expanded upon their work using directly visualized TBNI of cisplatin for endobronchial lesions to using the EBUS bronchoscope to deliver the agent into extrinsically located lesions. These authors reported a series of 50 sites treated with EBUS-TBNI of cisplatin in 36 patients. All patients had previously received full dose EBRT to hilar or mediastinal structures, had pathologically confirmed recurrence, and no evidence of distant metastasis. Forty milligrams (1 mg/mL) of cisplatin was delivered via EBUS-TBNI into the confirmed region of recurrence during four different procedures. It is notable that if multiple sites were treated the total dose per patient was limited to 40 mg (e.g., if there were two sites, each received 20mg). Transient nausea post-procedure was experienced by several patients. Two patients in the series received concomitant reirradiation and both developed bronchomediastinal fistulas. Based on RESIST criteria, the response rate was 69% (complete or partial) and was associated with both a significant increase in overall and progression-free survival compared to non-responders. As in any retrospective series, a randomized multicenter trial is needed to deal with potential confounding and selection bias. However, the response rate is remarkable given that most patients had previously received platinum-based chemotherapy and speaks to the ability to achieve higher concentrations within the tumor. In fact, when cisplatin is given intravenously, the concentration of drug taken up by the tumor is lower than that taken up by normal tissues (http://www.accessdata.fda.gov, accessed 2/1/17). Cisplatin injected directly into the tumor avoids this problem by allowing delivery of a high concentration of the agent. This high concentration may also overcome some resistance mechanisms of the tumor (21,22). The ability to achieve a higher intratumoral concentration likely also underlies the occurrence of bronchomediastinal fistula in both patients who received reirradiation, since cisplatin is a known radiation sensitizer. EBUS-TBNI is thus currently contraindicated in any patient who is receiving, or will potentially be receiving, radiation therapy.
We have also used EBUS-TBNI with cisplatin to alleviate symptomatic, extrinsic airway compression in regions previously radiated (Figure 1). No large series has reported on this indication but anecdotally it has been successful. The same general technique and dose are used. In these cases, EBUS-TBNI commonly serves an adjunct to airway stenting.
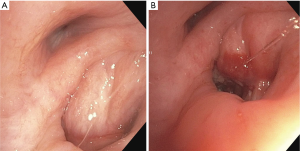
Importantly, “therapeutic” (based on IV dosing) cisplatin blood levels have been recorded following EBUS-TBNI delivery of total doses ranging from 30 to 40 mg intratumorally. Given the high rate of adverse events in the series by Celikoglu in patients receiving concomitant reduced dose intravenous chemotherapy and the blood levels we have noted, it is likely that 40mg is close to the maximally tolerated dose. Taken together these data imply that delivering cytotoxic agents via EBUS-TBNI is contraindicated for patients receiving concomitant intravenous cytotoxic therapy.
Current challenges and opportunities
There remain significant unanswered questions regarding EBUS-TBNI delivered therapies. Many of the questions posed in an editorial by Ost and Sterman with regard to directly visualized TBNI are also relevant for EBUS-TBNI (23). Perhaps the most obvious is that of dosing of the agent. However, further consideration reveals the complexity associated with this seemingly straightforward question. Even for the most commonly used drug, cisplatin, dosing remains unclear. Although 40mg appears to approach the maximum tolerated dose this may vary by patient, and more specifically, within a given tumor structure. There is evidence of significant heterogeneity among collagen density, blood flow, interstitial fluid pressure, and malignant cell density within the structure of a tumor (24). Thus the given distribution of an agent may depend on a number of interrelated features including the structure of the tumor, the vascular supply which serves as a “sink”, the diffusion coefficient of the agent, and the method of delivery (e.g., the number and location of injections). These considerations are likely important for delivery of any aqueous or semi-solid agent.
An area of significant opportunity is in the identification and development of alternative agents for EBUS-TBNI. In addition to a number of potentially effective cytotoxic agents, there is considerable interest in local immunotherapy for lung cancer (25). The efficacy of intratumoral therapy with oncolytic viruses has been demonstrated for a number of other cancers, most notably melanoma, where the herpes virus mediated T-vek is now FDA approved (26). In an interesting Phase I study, Lee and co-workers used directly visualized TBNI to deliver CCL21 expressing dendritic cells intratumorally for a subset of patients (27). This chemokine is important for lymphoid organs and potentially may augment the immune response. Similarly trials of intratumoral oncolytic viruses in addition to immunotherapy are ongoing (25), although the delivery method remains unclear.
Further, the application of EBUS-TBNI for small cell lung cancer, other thoracic malignancies, lymphomas, and metastatic disease is unknown. Given the unparalleled access to the airways and the wide potential selection of therapies, there is significant opportunity for therapeutic applications in these and other lung diseases.
Conclusions
The power of EBUS-TBNA for diagnosis and staging of lung cancer has led to significant interest in applying this technology therapeutically. Until prospective studies that directly consent patients for potential risks, including bronchomediastinal fistula, mediastinitis, and systemic toxicities, are performed EBUS-TBNI is contraindicated if a patient is receiving concomitant systemic therapy (or radiation. Similarly, there is no evidence of efficacy to support first-line therapy with EBUS-TBNI. However, there is early evidence to support the use of EBUS-TBNI delivered cisplatin for treatment of locoregionally recurrent lung cancer in a previously radiated field. Despite previously receiving cisplatin-based chemotherapy, the response rates remain relatively high. Further studies are needed to establish the evidence base necessary for this therapy to be a recommended approach for locoregionally recurrent disease.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Amit Mahajan, Sandeep J. Khandhar and Erik E. Folch) for the series “Management of Complex Airway and Pleural Diseases” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.06.05). The series “Management of Complex Airway and Pleural Diseases” was commissioned by the editorial office without any funding or sponsorship. The author serves as a consultant for Olympus Respiratory America. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non–small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol 2000;18:2354-62. [Crossref] [PubMed]
- Yasufuku K, Chiyo M, Sekine Y, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration of mediastinal and hilar lymph nodes. Chest 2004;126:122-8. [Crossref] [PubMed]
- Tanner NT, Pastis NJ, Silvestri GA. Training for Linear Endobronchial Ultrasound Among US Pulmonary/Critical Care FellowshipsTraining for Linear Endobronchial UltrasoundA Survey of Fellowship Directors. Chest 2013;143:423-8. [Crossref] [PubMed]
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive SummaryExecutive SummaryDiagnosis and Management of Lung Cancer: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:7S-37S. [Crossref] [PubMed]
- Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J 2009;33:1156-64. [Crossref] [PubMed]
- Eapen GA, Shah AM, Lei X, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration: Results of the AQuIRE registry. Chest 2013;143:1044-53. [Crossref] [PubMed]
- Asano F, Aoe M, Ohsaki Y, et al. Deaths and complications associated with respiratory endoscopy: a survey by the Japan Society for Respiratory Endoscopy in 2010. Respirology 2012;17:478-85. [Crossref] [PubMed]
- Amini A, Lou F, Correa AM, et al. Predictors for locoregional recurrence for clinical stage III-N2 non-small cell lung cancer with nodal downstaging after induction chemotherapy and surgery. Ann Surg Oncol 2013;20:1934-40. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer 2006;94:1099-106. [Crossref] [PubMed]
- Fan C, Gao S, Hui Z, et al. Risk factors for locoregional recurrence in patients with resected N1 non-small cell lung cancer: a retrospective study to identify patterns of failure and implications for adjuvant radiotherapy. Radiat Oncol 2013;8:286. [Crossref] [PubMed]
- Nichols L, Saunders R, Knollmann FD. Causes of Death of Patients With Lung Cancer. Arch Pathol Lab Med 2012;136:1552-7. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Rulach R, Hanna GG, Franks K, McAleese J, Harrow S. Re-irradiation for Locally Recurrent Lung Cancer: Evidence, Risks and Benefits. Clin Oncol (R Coll Radiol) 2018;30:101-9. [Crossref] [PubMed]
- Stout R, Barber P, Burt P, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol 2000;56:323-7. [Crossref] [PubMed]
- McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819-27. [Crossref] [PubMed]
- Seymour CW, Krimsky WS, Sager J, et al. Transbronchial needle injection: a systematic review of a new diagnostic and therapeutic paradigm. Respiration 2006;73:78-89. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Treatment of Malignant Airway Obstruction with Intratumoral Injection of Chemotherapy with Cisplatin. Ann Am Thorac Soc 2015;2015:150613193717008 [PubMed]
- Spiro SG, Rudd RM, Souhami RL, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 2004;59:828-36. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Niu Q, Wang W, Li Q, et al. Percutaneous Fine-Needle 5% Ethanol-Cisplatin Intratumoral Injection Combined with Second-Line Chemotherapy Improves On the Standard of Care in Patients with Platinum-Pretreated Stage IV Non-Small Cell Lung Cancer. Transl Oncol 2014;7:303-8. [Crossref] [PubMed]
- Kim ES, Lee JJ, He G, et al. Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol 2012;30:3345-52. [Crossref] [PubMed]
- Ost DE, Sterman DH. Interventional Bronchoscopy in 2015. Removing Endoluminal and Methodological Obstructions. Ann Am Thorac Soc 2015;12:1265-6. [Crossref] [PubMed]
- Jain RK, Martin JD, Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 2014;16:321-46. [Crossref] [PubMed]
- Murthy V, Minehart J. the DS. Local immunotherapy of cancer: innovative approaches to harnessing tumor-specific immune responses. J Natl Cancer Inst 2017;109. [PubMed]
- Andtbacka RHI, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 2015;33:2780-8. [Crossref] [PubMed]
- Lee JM, Lee MH, Garon E, et al. Phase I trial of intratumoral injection of CCL21 gene modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8+ T cell infiltration. Clin Cancer Res 2017;23:4556-68. [Crossref] [PubMed]
Cite this article as: Kinsey CM. Endobronchial ultrasound-guided-transbronchial needle injection for direct therapy of lung cancer. AME Med J 2018;3:74.

