Establishing a lung cancer screening program in a non-university hospital
Introduction
Lung cancer is the most common cause of cancer death both in the United States and worldwide (1). Unfortunately, the majority of lung cancer is diagnosed in late stages leading to a poor five-year survival (2). Unlike other leading causes of solid tumor cancers (breast, colon, and cervix), until recently, there was no adequate modality for lung cancer screening (LCS). In the last decade, low dose CT scans have become the standard of care for LCS and are associated with 20% reduction in mortality (3). Unfortunately, only about four percent of eligible patients in the United States receive appropriate LCS (4). Proper LCS requires a well-organized program (5). In this article, we identify the critical components of a LCS program, with particular focus on those central to a non-university hospital setting. Moreover, we will highlight a number of controversies and specific challenges and will draw upon the experience we garnered from establishing and growing our own program.
Importance of non-university LCS programs
The National Lung Screening Trial (NLST) enrolled 53,454 study subjects between August 2002 and April 2004, screening with either plain chest radiography or low-dose non-contrast computed tomography (CT) (3). This landmark study, published in 2011, demonstrated a clear reduction in mortality, both lung cancer specific and overall, among those at high-risk for the disease. Ultimately, this resulted in a grade B recommendation from the USPSTF (6). In turn, and even before the Centers for Medicare Services (CMS) and private insurance carriers agreed to cover the service, providers in the community setting began offering lung cancer CT screening. However, replication of the positive results of the NLST are contingent upon following similar practices for subject selection, radiographic technique, study interpretation, diagnostic decision-making, and procedural reliability.
Most of the 33 participating centers in the NLST were either National Cancer Institute (NCI) designated hospitals or large research-oriented academic systems. Moreover, the vast majority of lung cancer diagnosis and treatment occurs in a community hospital setting (7). These two points together are critically important as academic and community hospitals may be different with regard to access to care and reproducibility of diagnostic and therapeutic results.
Non-university health systems may have advantages in both adaptation of screening for eligible persons, and adherence to established protocols once enrolled. The patients’ primary care physicians (PCPs), who are based in the community, are often best equipped to recognize eligibility and recommend LCS. Patients have an initial level of trust in the providers with whom they are familiar. Electronic medical record systems can be leveraged to identify eligible patients. Additionally, a non-university community health system already has an established network for marketing and public service messaging that reaches the population in their homes and workplaces. Known hurdles such as time, transportation, and out-of-pocket expenses are all lessened when care is delivered locally.
When a positive finding is seen on screening, patients may receive further diagnostics and treatment at a non-university community hospital, or may be referred to a larger center. Those non-university hospitals with the proper resources and systems may have certain advantages related to easier access, potentially resulting in more timely diagnosis and treatment. However, non-university community health systems have particular challenges which introduce variability into the equation. First, physicians of various specialties may not work in a coordinated fashion. Second, there may be disparate medical record systems which act as a barrier to effective and efficient communication. Additionally, there may not be an established multidisciplinary conference that meets with appropriate frequency. Moreover, a dedicated thoracic radiologist may not exist. Additionally, state-of-the-art diagnostic technologies may be unavailable and minimally-invasive surgery for early-stage cancers may not be offered if the local surgeons do not possess that skillset. Consequently, surgical results may not be measured against national benchmarks, especially if a board-certified thoracic surgeon is not a programmatic component. Although any of these pitfalls may also exist at a larger center, it is the non-university community hospitals which are generally most vulnerable. In order to simulate the NLST results, these challenges need to be overcome.
Stamford Health is a mid-size hospital system located in a suburb of New York City consisting of a 305-bed hospital, multiple outpatient facilities, three radiology entry sites, and medical staff of roughly 700 physicians. We have a LCS program which has evolved over the past five years and is accredited by the American College of Radiology and designated as a Center of Excellence by the Lung Cancer Alliance. Our program performs approximately 400 LCS CT scans annually. We have managed to overcome many of these obstacles, yet some challenges remain.
Key elements for establishing a LCS program
Based on our experience, a well-designed program requires five key components: (I) program leadership; (II) radiology infrastructure; (III) standardized workflows, reporting, and data tracking; (IV) marketing and communication; and (V) reimbursement and finance. Most institutions already have some of this groundwork established, only not yet coordinated. Moreover, for a LCS program to succeed in the community, there is a need for constant reassessment and improvement of these key areas.
Program leadership
The management of lung cancer has long relied on a multidisciplinary team to provide optimal treatment. In best practice, this team requires thoracic surgeons, medical oncologists, radiation oncologists, pulmonologists, pathologists, interventional and diagnostic radiologists, palliative care physicians, as well as nursing, social work, and nutrition (8). LCS requires a similar approach. The American College of Chest Physicians and the American Thoracic Society joint statement supports involving (I) pulmonary medicine, (II) diagnostic and interventional radiology, (III) thoracic surgery, (IV) medical oncology, and (V) radiation oncology (5). In our experience, as the goal of CT LCS is to identify early stage cancers that are appropriate for curative therapy, the key physician leadership should be from pulmonary medicine, thoracic surgery, and diagnostic radiology. Technical aspects of the scans as well as structured reporting are best managed by radiology leadership. However, direct communication with referring physicians and patients is often best handled by the clinical leadership from pulmonary and thoracic surgery, as they are well-positioned to recommend next steps of care based on the patient’s findings and physiological status. For non-university based programs, the importance of involvement of PCPs cannot be understated (9). As they are the gateway to the program, their needs, workflow, and specific challenges must be addressed. Appointing a respected primary care ambassador to the program can be very helpful to convince others to follow suit.
Without the support of hospital administration, a LCS program will not get off the ground. Understanding the economic impact of a LCS program for an institution is necessary to create a profit/loss estimate for the health system. There have been various models for financially optimizing programs recognizing that the greatest impact is creation of downstream revenue (10,11). Much of the investment is on the front end, such as nursing salary support, marketing dollars, software infrastructure, and possibly other diagnostic and treatment technologies. Success is best when the physicians and administrators work side by side toward a common goal.
Many LCS programs are housed in an organization’s cancer center, some in the radiology department, and others are based in thoracic surgery or pulmonary medicine. This decision is institutionally specific but any structure needs to account for the multidisciplinary nature of LCS.
Early LCS studies were performed in the context of research protocols with the associated resources of a research team. With more widespread adoption, nurse navigation has become a cornerstone of program leadership. Navigators have been shown to be central for the management of other cancer screening programs such as breast cancer and colorectal cancer (12,13). Specifically, navigators are critical for confirming patient eligibility, maintaining data systems, and encouraging patient follow-up with specialists when necessary. Studies have shown that the use of navigators increases the rate of screening in high-risk patient populations (14).
Radiology infrastructure
A series of very specific requirements for CT scan machines as well as radiologist qualifications must be met to qualify for CMS reimbursement (15). Some of these requirements can be particularly challenging for non-university hospitals. Most conventional CT scanners will satisfy the CMS requirement of 3 mGy dosing for standard-sized patients; nonetheless, it is incumbent upon the program leadership from radiology to ensure low-dose lung cancer CTs are protocolized appropriately. The bigger issue for many non-university programs is the diagnostic radiologist qualifications. Interpreting radiologists must be board-certified or board-eligible by the American Board of Radiology or equivalent organization and participate actively with Continuing Medical Education. But more specifically, CMS has mandated that a participating radiologist interpret at least 300 chest CT studies in the past 3 years. This may limit which radiologists at an institution are permitted to read LCS CTs. In our institution, to insure consistency and accountability, we limited interpretations for LCS CTs to a small team of qualified and interested radiologists led by our program’s radiology director.
As part of the 2015 CMS Decision Letter, LCS programs are required to report data to an approved lung cancer registry (15). Since 2015, the only approved data reporting mechanism is the American College of Radiology (ACR) Lung Cancer Screening Registry. Non-university hospitals should become familiar with this registry prior to developing a LCS program (16).
Standardized workflow, reports, and data management
A successful LCS program requires a standardized workflow for navigating patients from recognition of eligibility through enacting recommendations based on scan results. Our program identified four key points in the workflow for the patient: (I) guidelines for patient eligibility; (II) shared decision-making visits; (III) ordering the appropriate CT scan; (IV) CT scan reporting.
Over the past several years, a number of international societies have released slightly different guidelines for LCS. Taken together, the major recommendations have centered on patients between 50 and 80 years old with 20 to 30 pack-year smoking history who are either active smokers or quit within the past 10–15 years. Because the majority of our patients have Medicare, we follow the CMS guidelines mandating patients to be 55–77 years old, at least 30 pack-year smokers, either active smoker or having quit within the past 15 years (15). Our team feels it is important to remain consistent with eligibility requirements, as there will be constant requests for screening patients who do not qualify. Answers to these requests should be scripted, with exceptional patients referred to a pulmonologist if other risk factors justify.
The use of electronic medical record (EMR) systems to identify eligibility and trigger referral for LCS is fraught with logistical challenges. For other solid tumor screening recommendations, use of EMR triggers has been low and complicated to implement (17). For LCS, identification of smoking history is the first hurdle. There is no standard way to record smoking history across EMRs, let alone within a single system. In fact, most EMRs have a “free text” box to detail smoking history with no structured format. Incorrect information has been a major obstacle to use EMRs to identify patients eligible for LCS. In a non-university community program, with multiple referring providers utilizing multiple EMRs, we have not been able to adequately identify patients appropriate for LCS.
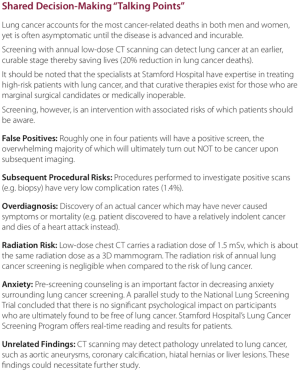
CMS has additional strict requirements for CT LCS (15). First, patients cannot self-refer for a LCS CT. Second, the initial order for a LCS CT must be furnished by a physician (or qualified provider such as physician’s assistant or nurse practitioner). The initial visit must also include documentation of a shared decision involving a thorough discussion of benefits and harms before initiating LCS. There are a number of decision aids available to help providers meet this requirement. As part of starting an LCS program, any provider ordering a LCS CT should be provided these decision aids. Our program has included one such aid in packets we provide to physicians (Figure 1).
The CMS guidelines for the written order for a LCS CT are quite explicit (15). In the CMS Decision memorandum the requirements are laid out specifically:
Written orders for both initial and subsequent LDCT lung cancer screenings must contain the following information, which must also be appropriately documented in the beneficiary’s medical records:
- Beneficiary date of birth;
- Actual pack-year smoking history (number);
- Current smoking status, and for former smokers, the number of years since quitting smoking;
- Statement that the beneficiary is asymptomatic (no signs or symptoms of lung cancer); and;
- National Provider Identifier (NPI) of the ordering practitioner.
PCPs have consistently noted that a major barrier to implementation of LCS is overly burdensome documentation which must be performed during a single appointment already consumed by the concomitant medical complexity of screening-eligible patients (9). These barriers to workflow must be minimized lest enrollment suffer. To make matters worse, most PCPs are not aware of the CMS documentation requirements to justify eligibility and reimbursement. In order to assist the PCPs, we created a standardized prescription form for all providers, either a paper or an electronic version that can be integrated into an EMR (Figure 2). This form contains all of the required information, no more and no less.
A standardized structured CT report is paramount for communicating with ordering providers, determining an appropriate management plan, and tracking data. Initially, our program used a modified version of the National Comprehensive Cancer Network guidelines (18). The ACCP/ATS recommendations strongly support using a structured reporting system as well as a nodule management algorithm (5). Our program subsequently adopted the American College of Radiology Lung RADS system in 2014 (19). This system has clear evidence-based recommendations that meet both of the above components.
Initially, our program utilized a simple spreadsheet under lock and key to manage patient findings and communicate with providers. As the number of patient scans increased, this type of system was not adequately sophisticated and provided undue work on our navigators. There are a number of commercially available software programs that can be used to facilitate data tracking and generate follow-up recommendations and communications. We recommend, prior to implementing a LCS program, an interdisciplinary meeting with information technology at the hosting institution to assess existing resources and identify the need for new software.
Marketing and communication
Marketing an LCS program should be viewed as an important public health service. Effective marketing consists of a two-pronged approach: (I) communicating the value of screening to referring physicians and (II) increasing awareness of the benefits of screening to the general public.
An annual chest radiograph had been a common phenomenon within the daily practice of PCPs. Early attempts of LCS using chest X-rays were unsuccessful (20), and the chest X-ray as part of an annual physical has faded away for the most part. This resulted in a relatively nihilistic attitude toward the early detection of lung cancer. In turn, this mindset has become a barrier to the adoption of the low-dose CT scan for LCS (21,22). In order to overcome this hurdle, a comprehensive plan of marketing and communication is essential. However, this can be challenging in a setting comprised of a grassroots primary care base with no clear mechanism for widespread communication and structured oversight.
From our experience, perhaps the most effective way to educate the primary care community is face-to-face. Hospital grand rounds presentations are certainly worthwhile for attending, resident, and student education, but the reality is most PCPs never make it to these conferences because they are overburdened with patient-care responsibilities in their offices. Much more useful, although time-consuming, is sending out program leadership to busy offices (usually with lunch) to concisely review the data supporting screening, stressing that it is now a standard-of-care akin to mammography and colonoscopy. Sharing success stories of their local colleagues who identified patients with early-stage cancers ultimately receiving curative therapy can be very impactful. We also found it helpful to provide a glossy “lung cancer screening kit” including laminated cards listing eligibility requirements and shared decision-making talking points, reprints of the NLST trial manuscript, as well as pre-printed prescription forms (Figure 1). Hospital-based newsletters and email blasts are other cost-effective ways to penetrate the physician community. Other specialists who see a large number of eligible patients include pulmonologists, cardiologists, and vascular surgeons.
Marketing a screening program to the community at-large can be costlier and more time-consuming. Television, radio,and billboards are often too expensive and ineffectively targeted. Direct mailings can be targeted to specific age groups, and in fact can be narrowed down to smokers as long as that data is available in an EMR. In fact, for lung cancer there is evidence this method has been effective (23). Patient education seminars can be held at retirement communities or even war veterans’ groups where smoking is prevalent. A robust website where search engine exposure is maximized can also drive self-referrals. Many hospital systems are taking advantage of social media platforms, as they are less expensive to leverage and have the ability to target specific demographics. Unfortunately, these platforms do not penetrate Baby Boomers as well as they do Generation X and Millennials. However, these eligible patients have children who may encourage their parents to seek screening.
Reimbursement and finance
When many LCS programs were first established, reimbur-sement for screening CTs was unclear and often confusing. If ordered as a diagnostic CT, either for a clinical problem such as cough or dyspnea, or to follow-up a lung nodule, patients are frequently subject to a co-payment. And without a consistent process in place, screening scans would mistakenly be performed as full-dose diagnostic studies. Thus, many LCS programs offered low-dose scans free of charge or at a nominal cost with the hope that downstream revenue would justify the expense (11). With the USPSTF recommendation and ultimately the CMS ruling endorsing screening for lung cancer, low-dose screening CTs are now covered as part of preventive healthcare (15). Patients, as well as healthcare providers, need to be aware at the time they have a shared decision visit, that while the CT scan is covered as preventative healthcare, follow-up testing and treatment (i.e., subsequent diagnostic studies, biopsies, or surgery) are not covered in the same manner (24). Avoiding confusion on this matter early-on is critical. As the majority of billing and reimbursement issues are related to the actual CT scan, we recommend a dedicated individual in the radiology department to help answer these questions for patients.
Program challenges
As LCS is still in its infancy, there is no singular optimal way to organize and manage a program. All programs are learning and correcting course as they mature, and more changes will occur as we gain experience and accumulate data. Here we will review how we handle specific issues and challenges with the intent of maximizing the benefit to our community.
Patient program “Point of Entry”
In order for LCS to be impactful, it is necessary to screen a maximal number of patients and treat them in a consistent and reliable manner. Other solid tumor screening programs such as breast cancer, colon cancer, and cervical cancer, can offer helpful lessons (25). However, the fundamental difference between these other cancer screening tests and low-dose LCS is the CMS mandate for a shared decision-making visit with a licensed practitioner. Although this is a well-intentioned rule, it can also act as a significant barrier to access, as reporting requirements are strict and not all PCPs are well-versed in the risks and benefits of screening (15). To satisfy this mandate, programs have adopted a number of different approaches (26).
Non-university programs must decide whether to offer a “single point of entry” or “multiple points of entry” into the program. A single-point of entry is usually through referral to a dedicated LCS clinic, and is most common in academic centers. It can be managed by either a physician or nurse practitioner. The benefit of this approach is that it is easier to satisfy all the requirements mandated by CMS and ensure strict quality controls for the program. The other approach, “multiple-points of entry,” allows any qualified practitioner to order low-dose screening CTs as long as they can satisfy and document the CMS requirements. This latter approach likely leads to better penetration of LCS in the community, as it does not necessitate additional appointments with associated travel and time from work. However, due to the non-centralized nature of this approach, program management and quality control can be more challenging. We have adopted a mixed approach allowing multiple points of entry for screening scans; however, should patients have high-risk findings, we strongly insist the subsequent management be limited to a dedicated team of specialists. As our program continues to expand, we are considering starting a parallel entry site allowing patients to self-refer to a nurse-practitioner staffed LCS clinic which will also satisfy all CMS requirements.
Specific patient eligibility
As a LCS program, we made a decision to follow the CMS guidelines for LCS. This decision presented a number of obstacles that we had to overcome. Prior to the formal CMS guidelines for LCS, multiple organizations had different guidelines for eligible programs. Our program initially used the NCCN guidelines that grouped patients in to high risk and moderate risk (18). The high risk patients included a group very similar to the CMS requirements (age 55–74, 30 pack-year smoker, and active smoker or quit within the last 15 years). However, NCCN allowed for a second group of patients who were 50 years old, had a 20 pack-year smoker, and had an additional risk factor such as second hand smoke, family history of lung cancer, or occupational exposures. With the CMS recommendations, our program moved to stop allowing these patients. Initially, we completed nodule management via our old guidelines; we now follow all patients via the ACR Lung RADS guidelines (19).
Patients who “aged-out” (greater than the age of 78) or “smoked-out” (quit more than 15 years prior) presented an additional obstacle. On the final year of a LCS “eligible” scan, many patients still have a lung nodule present. For these patients, all scans are reviewed by a program co-director and a recommendation to the ordering physician is given. If the patient has demonstrated stability of the nodules per the Fleischner Society guidelines (27), we recommend no further CT scans. If there are new nodules on the last scan, or nodules that had not shown stability, we recommend further scans be performed outside of our LCS program and the recommendations be made per Fleischner Society guidelines. Most importantly, we communicate with the ordering providers to the rationale for stopping screening via a dedicated program and offer any patient a visit with a pulmonologist should there still be confusion.
There are a few instances when patients may be appropriate to screen for lung cancer but they do not meet the CMS guidelines. Patients with significant asbestosis exposure (28) or alpha-1 antitrypsin deficiency are examples (29). Moreover, we are frequently asked by primary care doctors if cigar or pipe smokers should be screened within the context of our program given the increase risk of lung cancer in these patients (30). In all these situations, LCS via a low dose CT scan may be appropriate. However, we feel it should be done outside the context of the traditional program and offer patients a pulmonary medicine consultation should they wish to discuss.
Accountability of patient results and follow-up
As we struggled to allow a broad range of providers to refer patients to our LCS program, accountability for abnormal findings was an early challenge. In the NLST trial, almost 25% of CT scans were noted to have abnormalities (3). As LCS programs moved away from clinical trials, a large Veterans Health Administration cohort found >50% of patients outside of trials had pulmonary nodules that required follow-up. Moreover, almost 40% had incidental findings such as emphysema, coronary calcification, and thyroid nodules that required follow-up (31). At our own institution, we have found a similar rate of 65% of scans revealing nodules. All these abnormalities created a struggle for our program to ensure adequate follow-up of these results. While using a “multi-point” system of entry allowing all primary care providers in our community to refer patients, we had to strike a balance between respecting the autonomy of these providers and ensuring that patients do not fall through the cracks.
Fortunately, the use of a standardize reporting system and nurse navigation has facilitated appropriate follow-up. The Lung RADS system (19) has a very clear reporting structure for pulmonary nodules and a clear recommendation for follow-up scans. As a team, for all patients who have a LungRADS 4 finding, designated as “suspicious” by the American College of Radiology, the ordering provider receives a call from a program director within 48 hours of the scan. At that point, we attempt to set up an appointment with either a pulmonologist or thoracic surgeon within 7 days. Our nurse navigator takes an active role in facilitating these appointments and other necessary scans. For LungRADS 3 findings, designated “probably benign,” our nurse navigator works with the ordering physician to arrange the recommended 6-month follow-up CTs. For LungRADS 3 and 4, a letter is also sent to the patients directly with notification of the findings and need for follow-up. For Lung RADS 1 and 2, designated “normal,” referring physicians are sent reminders to order the next annual CT scan. Like most programs, we have limited resources dedicated to ensuring adequate follow-up. Focusing on the highest risk scans, greater than 95% of our LungRADS 4 patients and 80% of our LungRADS 3 patients complied with the appropriate recommended follow-up. Our system allows the ordering physician to communicate the scan results with their patients but from that point forward, our LCS program assumes the responsibility of follow-up for the highest risk patients. We find most PCPs appreciate this approach.
LungRADS designates incidental radiographic findings that are either clinically significant or potentially clinically significant as “S” findings. These are estimated by the ACR to be found in about 10% of all scans and we found similar findings in our cohort (19). For these findings, an alert is sent via our radiology department’s usual protocol to the ordering physicians. The most common findings are coronary calcification and thyroid nodules.
Many PCPs find the confusion and associated liability to be a disincentive to referring patients for LCS. Non-university LCS programs must contend with a decentralized set of electronic medical records and a non-affiliated autonomous primary care base. As such, we believe it is imperative for non-university programs to have a well-established set of protocols, and to assume the accountability for the surveillance and management of high-risk findings.
Conclusions
LCS has become the standard of care for appropriately selected patients. Unfortunately, only a very small fraction of eligible patients in the United States receive appropriate screening. To increase accessibility for patients, community non-university hospitals are critical to the mass adoption of LCS. In our experience, as well as other institutions, well-functioning LCS programs can be established in the community thus allowing more patients to reap the benefits of LCS.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ricardo Sales dos Santos, Myrna Godoy, Juliana Franceschini and Hiran C. Fernando) for the series “Update on Lung Cancer Screening and the Management of CT Screening Detected Pulmonary Nodules” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.10.01). The series “Update on Lung Cancer Screening and the Management of CT Screening Detected Pulmonary Nodules” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Lung and Bronchus Cancer 2016. Available online: https://seer.cancer.gov/statfacts/html/lungb.html, accessed September 10, 2018.
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States—2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- U.S. Public service Task Force. Lung Cancer: Screening Final Recommendation. Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening, accessed September 11, 2018.
- Miller DL, Mayfield WR, Luu TD, et al. Community-based multidisciplinary computed tomography screening program improves lung cancer survival. Ann Thorac Surg 2016;101:1864-9. [Crossref] [PubMed]
- Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev 2016;42:56-72. [Crossref] [PubMed]
- Triplette M, Kross EK, Mann BA, et al. An Assessment of Primary Care and Pulmonary Provider Perspectives on Lung Cancer Screening. Ann Am Thorac Soc 2018;15:69-75. [Crossref] [PubMed]
- Gilbert CR, Ely R, Fathi JT, et al. The economic impact of a nurse practitioner-directed lung cancer screening, incidental pulmonary nodule, and tobacco-cessation clinic. J Thorac Cardiovasc Surg 2018;155:416-24. [Crossref] [PubMed]
- McKee BJ, McKee AB, Flacke S, et al. Initial experiences with a free, high-volume, low-dose CT lung cancer screening program. J Am Coll Radiol 2013;10:586-92. [Crossref] [PubMed]
- Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of science. CA Cancer J Clin 2011;61:237-49. [Crossref] [PubMed]
- Freund KM. Implementation of evidence-based patient navigation programs. Acta Oncol 2017;56:123-7. [Crossref] [PubMed]
- Percac-Lima S, Ashburner JM, Rigotti NA, et al. Patient navigation for lung cancer screening among current smokers in community health centers a randomized controlled trial. Cancer Med 2018;7:894-902. [Crossref] [PubMed]
- Decision memo for screening for lung cancer with low dose computed tomography. Centers for Medicare and Medicaid Services. Available online: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274, accessed September 10, 2018.
- Lung Cancer Screening Registry. American College of Radiology. Available online: https://www.acr.org/Practice-Management-Quality-Informatics/Registries/Lung-Cancer-Screening-Registry, accessed September 10, 2018.
- Modin HE, Fathi JT, Gilbert CR, et al. Pack-Year Cigarette Smoking History for Determination of Lung Cancer Screening Eligibility. Comparison of the Electronic Medical Record versus a Shared Decision-making Conversation. Ann Am Thorac Soc 2017;14:1320-5. [Crossref] [PubMed]
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [Crossref] [PubMed]
- Lung‐RADS™ Version 1.0 Assessment Categories Release date: April 28, 2014. American College of Radiology. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads, accessed September 10, 2018.
- Brett GZ. The value of lung cancer detection by six-monthly chest radiographs. Thorax 1968;23:414-20. [Crossref] [PubMed]
- Rajupet S, Doshi D, Wisnivesky JP, et al. Attitudes About Lung Cancer Screening: Primary Care Providers Versus Specialists. Clin Lung Cancer 2017;18:e417-e423. [Crossref] [PubMed]
- Kanodra NM, Pope C, Halbert CH, et al. Primary Care Provider and Patient Perspectives on Lung Cancer Screening. A Qualitative Study. Ann Am Thorac Soc 2016;13:1977-82. [Crossref] [PubMed]
- Hinshaw LB, Jackson SA, Chen MY, et al. Direct mailing was a successful recruitment strategy for a lung-cancer screening trial. J Clin Epidemiol 2007;60:853-7. [Crossref] [PubMed]
- Eberth JM. Lung Cancer Screening With Low-Dose CT in the United States. J Am Coll Radiol 2015;12:1395-402. [Crossref] [PubMed]
- Armstrong K, Kim JJ, Halm EA, et al. Using lessons from breast, cervical, and colorectal cancer screening to inform the development of lung cancer screening programs. Cancer 2016;122:1338-42. [Crossref] [PubMed]
- Qiu R, Copeland A, Sercy E, et al. Planning and Implementation of Low-Dose Computed Tomography Lung Cancer Screening Programs in the United States. Clin J Oncol Nurs 2016;20:52-8. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Ollier M, Chamoux A, Naughton G, et al. Chest CT scan screening for lung cancer in asbestos occupational exposure: a systematic review and meta-analysis. Chest 2014;145:1339-46. [Crossref] [PubMed]
- Torres-Durán M, Ruano-Ravina A, Parente-Lamelas I, et al. Alpha-1 Antitrypsin Deficiency and Lung Cancer Risk: A Case-Control Study in Never-Smokers. J Thorac Oncol 2015;10:1279-84. [Crossref] [PubMed]
- Iribarren C, Tekawa IS, Sidney S, et al. Effect of cigar smoking on the risk of cardiovascular disease, chronic obstructive pulmonary disease, and cancer in men. N Engl J Med 1999;340:1773-80. [Crossref] [PubMed]
- Kinsinger LS, Anderson C, Kim J, et al. Implementation of lung cancer screening in the veterans health administration. JAMA Intern Med 2017;177:399-406. [Crossref] [PubMed]
Cite this article as: Bernstein MA, Ronk M, Ebright MI. Establishing a lung cancer screening program in a non-university hospital. AME Med J 2018;3:102.