Near-infrared spectroscopy predicts brain injury in patients on extracorporeal membrane oxygenation
Introduction
Extracorporeal membrane oxygenation (ECMO) is a supportive treatment used to temporarily support patients with severe heart or lung failure (1). Although ECMO supports cardiovascular and pulmonary function, neurological complications remain a leading cause of death in patients on ECMO (2-4). Neurological injury may be due to pre-existing anoxia related to pre-ECMO low flow states such as cardiac arrest prior to support, atherosclerotic diseases, ischemic stroke due to systemic embolization from the ECMO system, or intracerebral bleeding from anticoagulation needed for ECMO (5). Even knowing this, neurologic monitoring of ECMO patients remains challenging because most patients are deeply sedated for multiple reasons. Patients on ECMO may require paralytics due to pre-existing respiratory failure or have an open sternum due to post-cardiotomy failure. Other possible issues may arise if patients are on peripheral veno-arterial ECMO (VA ECMO), as there may be competing flow between native cardiac flow with poorly oxygenated blood from diseased lungs and retrograde ECMO flow with highly oxygenated blood from the ECMO circuit, which results in unknown cerebral oxygenation seen in conditions like “harlequin syndrome” or “north south syndrome” (6).
To ensure adequate cerebral perfusion, near infrared spectroscopy (NIRS) monitoring can be a modality of choice (7,8). NIRS detects regional oxygen level of brain tissue under light deflecting probes placed on the forehead of the patient and allows clinicians to monitor regional oxygen saturation trends on the right and left hemispheres (9). Previously, we advocated the use of NIRS as a routine monitoring method of cerebral perfusion, as it could detect regional ischemia and possible anoxic brain injury in ECMO patients (10). Although promising as a technique for measuring perfusion and possibly detecting intracranial injury, it may be prone to false positives or false negative NIRS readings (11,12). In particular, early ischemic events may not show direct changes in NIRS readings, and thus provide false negatives in the setting of acute strokes (13). In addition, possible disruptions can occur from patient transportation from the intensive care unit (ICU) to the computed tomography (CT) scan, which places a heavy burden on the staff, hospital resources, and especially the patient (14). In this paper, we would like to elucidate the accuracy (sensitive and specificity) of NIRS in detecting neurological injury by comparing and combining it to neurological examinations. In addition, the study will examine how well NIRS detects oxygenation changes based on the location of the insult.
Methods
With institutional review board approval (Thomas Jefferson University IRB #11D.185), patient demographics, ECMO data, and ECMO complications of patients who underwent either veno-arterial or -venous ECMO were prospectively entered into database from 2010 to 2017. Clinical signs and NIRS readings were used to examine suspected neurological injury. Patients were then sent to CT scan to confirm or rule out possible injury and these results were extracted from electric medical record (EMR). Those who did not receive a CT scan while on ECMO were excluded from this study.
NIRS monitoring was performed using INVOSTM (Somanetics/Covidien, Inc., Boulder, CO, USA) or FORE-SIGHTTM (CAS Medical Systems, Branford, CN, USA) since 2010. NIRS was routinely placed on all ECMO patients in our institution and continued while patient was on ECMO. This was done whether the patient was on veno-arterial or veno-venous ECMO. NIRS data of those who had a CT scan on ECMO were retrospectively obtained from EMR, of which the nursing staff manually entered each value every hour or every 2 hours. Those who were missing NIRS readings in their EMR were excluded from this study. The “baseline NIRS” reading was determined as the average of at least 6 hourly readings 12 hours prior to CT scan. The “event NIRS” was collected at the time of CT scan for each patient as well. The differences between NIRS readings were calculated and averaged. Patients who underwent a CT scan were grouped based on the indications of CT scan: those demonstrating clinical neurological signs with significant NIRS event, as documented in the physician’s progress note (Group A), those with neurological signs without NIRS event (Group B), those with NIRS event without neurological signs (Group C), and those without neurological signs or NIRS event (Group D). To identify the contribution of NIRS in detecting neurological injury, group comparison was performed. Groups were further divided into those with “coma despite sedation vacation of 24 hours” (COMA) or “acute neurological injury” (ANI), which was defined as a clinical neurological sign that included new onset hemiplegia, unequal pupils, and seizures. Neurological findings were determined by the ICU physicians caring of these patients.
Data was expressed as numbers with a percentage or mean ± standard deviation. Data comparisons between groups were performed with a Fisher’s chi-square test or double tailed Mann-Whiney U-test, appropriately. Sensitivity and specificity were calculated to evaluate if a NIRS event detected a CT confirming neurological injury and were expressed with 95% confident intervals (CI).
Results
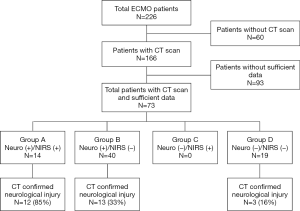
Between 2010 and 2017, 204 patients underwent ECMO and 166 patients (81%) had at least one CT scan while on ECMO. Among them, a total of 73 patients (36%) had appropriate NIRS documentation prior to CT scan to include in this study. The baseline characteristics of studied patients are shown in Table 1.
Table 1
| Number of ECMO patients | N=73 |
|---|---|
| Pre-ECMO demographics | |
| Age (years) | 49±13 |
| Male gender | 51 (70%) |
| Body surface area (cm2) | 2.0±0.3 |
| Time from ECMO to CT scan (days) | 4.4±4.6 |
| Type of ECMO | |
| Veno-arterial | 56 (77%) |
| Veno-venous | 17 (23%) |
| Indication for ECMO | |
| Cardiac ECMO | 39 (53%) |
| Respiratory ECMO | 24 (33%) |
| E-CPR | 10 (14%) |
| Detailed ECMO indication | |
| Acute myocardial infarction | 14 (19%) |
| Post-cardiotomy failure | 12 (16%) |
| Aspiration pneumonia | 7 (10%) |
| Acute on chronic heart failure | 6 (8%) |
| Malignant arrhythmia | 6 (8%) |
| Acute myocarditis | 5 (7%) |
| Viral pneumonia | 5 (7%) |
| Bacterial pneumonia | 3 (4%) |
| Other* | 15 (21%) |
Data are expressed as number with percentages or mean ± standard deviation. *, other includes interstitial pneumonia [2], takotsubo cardiomyopathy [2], pulmonary embolism [2], acute respiratory distress syndrome [2] septic shock [1], asthma [1], drug induced cardiac arrest [1], constrictive pericarditis [1], alveolar hemorrhage [1], fungal pneumonia [1], PCP pneumonia [1]. ECMO, extracorporeal membrane oxygenation; CT, computed tomography; E-CPR, ECMO assisted cardiopulmonary resuscitation; PCP, Pneumocystis pneumonia.
Based on the indications of CT scan, there were 14 patients (19%) who had clinical neurological signs and NIRS event (Group A), 40 patients (55%) who had clinical neurological signs only without NIRS event (Group B), and 19 patients (26%) who did not show any neurological signs or NIRS event (Group D) (Figure 1). None of the patients that underwent a CT scan demonstrated isolated NIRS event without neurological signs (Group C). The indications for a CT scans within Group D were retrospectively unclear (incidental) but were predominantly requested from referral physicians or taken because of necessity for other organ CT scans. For this reason, Group D was taken out of study considerations. The clinical neurological signs that prompted a CT scan were further categorized into COMA (45 patients, 62%) and ANI [unequal pupils (5, 7%), new onset of hemiplegia (3, 4%), and seizures (1, 1%)] as shown in Table 2. CT scans confirmed neurological injury in 28 patients (38%), including 12 patients (86%) in Group A, 13 patients (33%) in Group B, and 3 patients (16%) in Group D, (P=0.006).
Table 2
| Group A (N=14) | Group B (N=40) | P value | |
|---|---|---|---|
| Neurological signs | Yes | Yes | |
| NIRS drop | Yes | No | |
| Pre-ECMO demographics | |||
| Age (years) | 48±14 | 51±14 | 0.530 |
| Male gender | 8 (57%) | 29 (73%) | 0.287 |
| Time from ECMO to CT (days) | 6.4±4.9 | 5.3±4.9 | 0.473 |
| Type of ECMO | |||
| Veno-arterial | 13 (93%) | 28 (70%) | 0.085 |
| Veno-venous | 1 (7%) | 12 (30%) | 0.085 |
| Indication of ECMO | |||
| Cardiac ECMO | 8 (57%) | 20 (50%) | 0.645 |
| Respiratory ECMO | 3 (21%) | 16 (40%) | 0.210 |
| E-CPR | 3 (21%) | 10 (10%) | 0.788 |
Data are expressed as number with percentage or mean ± standard deviation. NIRS, near infrared spectroscopy; ECMO, extracorporeal membrane oxygenation; CT, computed tomography; E-CPR, ECMO assisted cardiopulmonary resuscitation.
Comparing the baseline data between group A and B (group C had no patients), there were no noteworthy differences between these groups except PaO2 and heart rate (Table 3). The average NIRS difference from baseline was −4.7±4.1 on the right and −5.1±3.9 on the left for group A and B combined. In group A, NIRS difference was −12.6±6.4 on the right and −11.9±4.1 on the left vs. −0.1±3.5 and −0.4±5.1 in Group B, excluding 2 patients with consistently low NIRS in group A (Table 2). These two patients started ECMO with NIRS persistently below 30 and were included in Group A but not for NIRS event calculations. The average reading between left and right NIRS reading at the time of CT scan was −11.6±7.6 in Group A, −0.3±6.2 in Group B, P<0.001. These values are also listed in Table 2.
Table 3
| Group A (N=14) | Group B (N=40) | P value | |
|---|---|---|---|
| Neurological signs | 14 (100%) | 40 (100%) | |
| COMA (coma despite 24 vacation) | 12 (86%) | 33 (83%) | 0.781 |
| ANI (acute neurological injury) | 2 (14%) | 7 (18%) | 0.782 |
| Pupils size issue | 1 (7%) | 4 (10%) | 0.751 |
| Hemiplegia | 1 (7%) | 2 (5%) | 0.763 |
| Seizure like activity | 0 (0%) | 1 (3%) | 0.550 |
| Patient condition | |||
| Temperature (oF) | 96±3 | 98±2 | 0.024 |
| Heart rate | 74±24 | 98±22 | 0.002 |
| Respiratory rate | 14±5 | 14±8 | 0.954 |
| Mean arterial pressure (mmHg) | 86±12 | 80±12 | 0.092 |
| Glasgow coma scale | 3.4±1.6 | 4.2±2.0 | 0.139 |
| Systemic oxygen saturation (%) | 95±5 | 95±4 | 1.000 |
| ECMO flow (L/min) | 4.1±0.7 | 4.5±0.9 | 0.116 |
| ECMO FiO2 (%) | 70±19 | 70±21 | 0.987 |
| Ventilator FiO2 (%) | 54±10 | 54±14 | 0.776 |
| pH | 7.37±0.07 | 7.40±0.06 | 0.159 |
| PaCO2 (mmHg) | 41±6 | 41±5 | 0.956 |
| PaO2 (mmHg) | 151±85 | 94±40 | 0.019 |
| NIRS reading | |||
| Baseline NIRS (right) | 56±11 | 64±11 | 0.023 |
| Baseline NIRS (left) | 56±12 | 65±10 | 0.015 |
| NIRS difference (right) | −12.6±6.4 | −0.1±3.5 | <0.001 |
| NIRS difference (left) | −11.9±4.1 | −0.4±5.1 | <0.001 |
| NIRS “event” difference* | −11.6±7.6 | −0.3±6.2 | <0.001 |
| Positive CT findings | 12 (86%) | 13 (33%) | 0.001 |
| Ischemic stroke | 5 (36%) | 6 (15%) | 0.098 |
| Anoxic brain injury | 4 (29%) | 1 (3%) | 0.004 |
| Intracranial bleed | 3 (21%) | 6 (15%) | 0.579 |
Data are expressed as number with percentage or mean ± standard deviation. *, NIRS “event” difference is the difference between the right and left NIRS reading at time of CT scan, indicating unilateral NIRS drop. NIRS, near infrared spectroscopy; ECMO, extracorporeal membrane oxygenation; CT, computed tomography.
Sensitivity and specificity of detecting a CT confirming neurological injury based on clinical indications were 36% (95% CI, 22–51%) and 89% (95% CI, 72–98%). Sensitivity and specificity in group A was 43% and 96% vs. 46% and 40% in Group B (Table 4). Among COMA patients (45/54, 83%), the sensitivity and specificity of detecting a CT scan confirming neurological injury based on NIRS event was 59% (95% CI, 33–82%) and 93% (95% CI, 77–99%).
Table 4
| Patients | CT confirmed | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|
| Group A + B combined | 54 | 25 (46%) | 36% (22–51%) | 89% (72–98%) |
| Group A: neuro signs (+), NIRS drop (+) | 14 | 12 (85%) | 43% (25–63%) | 96% (85–99%) |
| Group B: neuro signs (+), NIRS drop (−) | 40 | 13 (33%) | 46% (28–66%) | 40% (26–56%) |
| COMA* Group (combined A & B) | 45 | 17 (38%) | 59% (33–82%) | 93% (77–99%) |
Data are expressed as number with percentage or sensitivity, specificity with 95% CI. *, COMA is defined as coma for 24 hours despite sedation vacation. CI, confident interval; NIRS, near infrared spectroscopy; CT, computed tomography.
Group A and B were divided into subsets of those with COMA or ANI diagnoses. In CT positive patients specifically, 10/12 (83%) patients in Group A had COMA while 7/13 patients (54%) in Group B had COMA. The distribution of strokes in COMA subset ad ANI subset of Group A and B are listed in Table 5. Group B’s ANI subset was grouped together as non-anterior cerebral artery (ACA)/middle cerebral artery (MCA) in Table 5 for simplicity. The sensitivity and specificity of detecting a neurological injury in the frontal temporal region (ACA/MCA) region, which is expected given the placement of NIRS equipment, is 82% (95% CI, 48–98%) and 79% (95% CI, 49–95%) respectively.
Table 5
| Group A CT+ (N=12) | Group B CT+ (N=13) | P value | |
|---|---|---|---|
| COMA | 10 (83%) | 7 (54%) | 0.114 |
| Anterior or middle cerebral artery | 4 | 2 | 0.628 |
| Posterior cerebral artery | 2 | 2 | 0.682 |
| Anoxic brain injury | 4 | 0 | 0.056 |
| Multiple stroke locations | 0 | 3 | 0.022 |
| Acute neurological injury (ANI) | 2 (17%) | 6 (46%) | 0.114 |
| Anterior or middle cerebral artery | 1 | 0 | 0.064 |
| Non-ACA/MCA* | 1 | 6 | 0.064 |
*, non-ACA/MCA include territories of the deep temporal artery, basilar artery, or cerebellar arteries. ACA, anterior cerebral artery; MCA, middle cerebral artery; ANI, acute neurological injury; CT, computed tomography.
Discussion
Altered neurological status is a concern for possible neurological injury because it may determine a patient’s outcome, and a CT scan is usually indicated to rule out possible brain injury. As we expected, concrete neurological signs such as those grouped in the ANI subset was likely to elicit a confirmed neurological injury via CT scan. In the case of NIRS event, our institutional protocols are to prompt CT scans for unilateral NIRS events and start bedside interventions for bilateral drop (6). These interventions are an attempt to increase cerebral perfusion, such as by increasing PaO2 or increasing systemic blood pressure, and this may have led to statistically significant differences in those values between groups A and B seen in Table 2. However, this protocol was not always followed, as a NIRS event would often prompt physicians to perform a clinical neurological exam before a CT scan was performed, thus moving many potential patients from Group C to Group A after confirmation of clinical neurological signs.
Previously we used a 20% NIRS drop as an indication for neurological work up based on previous NIRS studies in both cardiac surgery and non-cardiac surgery settings (6,15). Interestingly, the practice was set to prompt a CT scan if a 20% saturation drop was observed, yet only 3 patients demonstrated greater than a 20% NIRS drop based on nursing documentation and physicians decided to take patients for a CT scan at 10–15% NIRS drop instead. This may be due to physicians being more judicious by using a stricter criteria of NIRS drop in conjunction with neurological signs or may be related to a data harvesting issue, as our study’s baseline and event calculation averages led to relatively more stable readings than studies that used a single hourly saturation difference. Additionally, it is important to consider the inclusion criteria that the study used may have excluded patients with serious neurological complications merely due to insufficient data or lack of CT scans due to decompensation. Patients who started ECMO at another institution may also not have had sufficient data at our institution, and may not have fit our study design of using the first CT scan available.
In our review, we chose to divide the patients into four groups based on the clinical indications for a CT scan listed in the physician notes. This grouping, however, is also prone to selection bias, as many notes are not uniform and may contribute to different results based on how physicians chart their patients and how data recording is formatted. Including patients with a CT scan as part of the inclusion criteria also presents its own selection bias for those with neurological injury and future studies should include ECMO patients with NIRS documentation without CT scans. Our study also demonstrated a significant number of patients that underwent a CT scan without any neurological sign, as seen in Group D. Among those who were without neurological signs, only a small number of the patients had positive CT findings (16%) compared to those that had neurological signs (25/54, 46%). Group D could represent a lack of uniformed guideline for CT scans for patients on ECMO.
Our study demonstrated a greater specificity for positive CT scans in patients with neurological change in conjunction with a cerebral saturation change as shown in Group A, demonstrating that a neurological exam itself is neither sensitive nor specific enough to detect a positive CT finding. Based on this difference between group A and B, NIRS event can provide additional information to prompt a CT scan in ECMO patients, and physicians will be better equipped to utilize CT scans in conjunction with NIRS trends. However, due to difficulties with charting the exact timing of neurological injury in relation to NIRS drop and presentation of clinical neurological symptoms along with the retrospective nature of this study, the sensitivity and specificity of this study should be evaluated with caution.
In terms of subgroups, COMA diagnoses did not necessarily lead to positive CT findings. This diagnosis may be more difficult in ECMO settings due to the need for deep sedation in these patients. Our protocol suggests that a CT scan is indicated if a patient was comatose for longer than 24 hours after sedation vacation. Although this was the most common indication for a CT scan, the results were often negative. Our study showed that among 45 COMA patients, only 17 patients (38%) had positive CT findings. Some patients may have come out of a coma after 24 hours due to slow clearance of sedatives associated with liver or kidney injuries sustained during the peri-ECMO period or sequestration of sedatives via the ECMO circuit, and this may have led to an inaccurate diagnosis of a “coma” (16). Comatose patients on ECMO may need to be observed over 24 hours or providers may need to wait for indirect signs of neurological injury such as a NIRS drop or ANI before clinicians decide whether there is a need for a CT scan. For these COMA patients in our study, it seems that NIRS may more reliable in revealing positive CT findings. NIRS changes may be of particular importance in comatose patients, whom clinicians will often encounter in patients on ECMO.
Despite NIRS’ noninvasive and clinically useful monitoring capabilities, it has yet to be widely adopted in many clinical practices (7,10,17). Even in our study, Group C had no patients because physicians used NIRS as an adjunct to the standard neurological exam, and did not obtain a CT scan for a saturation drop alone until a neurological exam was performed. Isolated NIRS drop without neurological findings may be related to low cerebral oxygenation and may be correctable through an increase of blood pressure, oxygenation, hemoglobin and ECMO flow, while maintaining an appropriate level of sedation. Future prospective studies into the viability of using cerebral saturation drop, especially without a neurological exam, are needed.
As stated earlier, NIRS sensors are placed on the forehead and will be expected to predominantly detect ACA and possibly MCA distributions of injury. Based on the study, this appears to affirm prior knowledge, as the sensitivity and specificity were relatively high in the expected distribution (82%, 79% respectively). When placed appropriately, NIRS is able to detect global cerebral hypoperfusion, particularly in the ACA and MCA region, and thus could give an earlier prediction of anoxic brain injury. However, given the poor prognosis of anoxic brain injury, it is unclear whether early diagnosis can lead to improved outcomes (18). Commonly missed neurological injury sites included the cerebellum, basilar artery, and injuries that involved multiple strokes, which may have led to hyperperfusion compensation (19). A possible solution to this may be global NIRS monitoring with automated value inputs that would limit the issue of not detecting certain regions of the brain or inadequate NIRS documentation due to human error.
It should also be noted that NIRS itself has its own limitations, particularly with the contamination of the signal from extracranial tissue from skull and skin. This discrepancy may not be noteworthy as a review study into the application of NIRS in carotid and cardiac surgery, brain injury, and general anesthesia demonstrate that readings are not largely affected by this contamination, especially with its use as a trend monitor (9). A persistently low NIRS reading below 40 could be a sign of diffuse brain injury, so following the trend of NIRS and the difference between the left and right hemisphere may be more important than a one-time NIRS reading to diagnose neurological damage (7,20,21). Early NIRS use may not always show a classic NIRS drop, as studies have shown increased respiratory rate, arterial pCO2, and surrounding hyperperfusion can lead to skewed readings (22). This may be resolved by using region-specific NIRS probe placement in future studies to find exact area of ischemia or bleeding. Currently, there are few, if any, strict recommendations in the care of ECMO patients, both in adults and in children. The only difference in protocol is the use of anticoagulation in children in acute stroke setting, where early recognition is key, as is the case with most stroke guidelines (23).
As with most retrospective studies like ours, there is an inherent selection bias that can lead to subjective classification of patients. This study was also done at a single institution, which is limited in its applicability. However, a single institution study could also benefit findings, as most patients likely had more consistent level of care as opposed to multi-institutional studies with hospitals that may not be as experienced with more newly implemented technology like ECMO and NIRS. Many recent studies into non-invasive neuro-monitoring currently lack the adequate power to sufficiently serve as guidelines, and future studies at other institutions are needed before further conclusions regarding short- and long-term neuro-protection outcomes of ECMO patients can be drawn (24). These studies should focus on specific patient populations, adults or pediatric, and follow specific pathologies in order to create more concrete protocols that physicians can use in patient care.
Conclusions
Neurological injuries in patients on ECMO are common complications. The neurological exam, when paired with NIRS, may provide more information in the management and care of patients with neurological injury on ECMO than the neurological exam alone. The utility of NIRS in conjunction with ECMO may be best observed for patients who are in a coma, as many ECMO patients often demonstrate this clinical sign while on ECMO, however clinical signs are still reliable in detecting neurological injury. Lastly, there is a high degree of accuracy in the detection of strokes in the expected regions of the brain where NIRS is placed, as seen in previous studies.
Acknowledgments
The authors would like to thank the providers and personnel that assisted in taking care of patients in this study, along with Jefferson Medical College and the Department of Surgery at Jefferson.
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.01.02). HH serves as an unpaid editorial board member of AME Medical Journal from Jan 2019 to Jan 2021. The other authors have no conflicts of interest declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). With institutional review board approval(Thomas Jefferson University IRB #11D.185). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769-78. [Crossref] [PubMed]
- Peigh G, Cavarocchi N, Hirose H. Saving life and brain with extracorporeal cardiopulmonary resuscitation: A single-center analysis of in-hospital cardiac arrests. J Thorac Cardiovasc Surg 2015;150:1344-9. [Crossref] [PubMed]
- Xie A, Lo P, Yan TD, et al. Neurological complications of extracorporeal membrane oxygenation: A review. J Cardiothorac Vasc Anesth 2017;31:1836-46. [Crossref] [PubMed]
- Fletcher-Sandersjöö A, Thelin EP, Bartek J Jr, et al. Incidence, Outcome, and Predictors of Intracranial Hemorrhage in Adult Patients on Extracorporeal Membrane Oxygenation: A Systematic and Narrative Review. Front Neurol 2018;9:548. [Crossref] [PubMed]
- Lorusso R. Extracoporeal life support and neuologic complications: still a long way to go. J Thorac Dis 2017;9:E954-6. [Crossref] [PubMed]
- Chung M, Shiloh AL, Carlese A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygentation. ScientificWorldJournal 2014;2014:393258 [Crossref] [PubMed]
- Wong JK, Smith TN, Pitcher HT, et al. Cerebral and lower limb near-infrared spectroscopy in adults on extracorporeal membrane oxygenation. Artif Organs 2012;36:659-67. [Crossref] [PubMed]
- Pozzebon S, Blandino Ortiz A, Franchi F, et al. Cerebral near-infrared spectroscopy in adult patients undergoing veno-artieral extracorporeal membrane oxygenation. Neurocrit Care 2018;29:94-104. [Crossref] [PubMed]
- Ghosh A, Elwell C, Smith M. Cerebral near-infrared spectroscopy in adults: a work in progress. Anesth Analg 2012;115:1373-83. [Crossref] [PubMed]
- Highton D, Elwell C, Smith M. Noninvasive cerebral oximetry: is there light at the end of the tunnel? Curr Opin Anaesthesiol 2010;23:576-81. [Crossref] [PubMed]
- Schober P, Bossers SM, Schwarte LA. Clinical study intracranial hematoma detection by near infrared spectroscopy in a helicopter emergency medical service : practical experience. Biomed Res Int 2017;2017:1-6. [Crossref]
- Moubayed SP, Mourad M, Urken ML. What are the optimal monitoring techniques in head and neck microvascular reconstruction? ORL J Otorhinolaryngol Relat Spec 2016;78:241-4. [Crossref] [PubMed]
- Erdoes G, Rummel C, Basciani RM, et al. Limitations of current near-infrared spectroscopy configuration in detecting focal cerebral ischemia during cardiac surgery: an observational case-series study. Artif Organs 2018;42:1001-9. [Crossref] [PubMed]
- Fitzgerald DC, Darling EM, Cardona MF. Staffing, Equipment, monitoring consdierations fo rextracorporeal membrane oxygenation. Crit Care Clin 2017;33:863-81. [Crossref] [PubMed]
- Maslehaty H, Krause-Titz U, Petridis AK, et al. Continuous measurement of cerebral oxygenation with near-infrared spectroscopy after spontaneous subarachnoid hemorrhage. ISRN Neurol 2012;2012:907187 [Crossref] [PubMed]
- Shah AG, Peahota M, Thoma BN, et al. Medication complications in extracorporeal membrane oxygenation. Crit Care Clin 2017;33:897-920. [Crossref] [PubMed]
- Trafidło T, Gaszyński T, Gaszyński W, et al. Intraoperative monitoring of cerebral NIRS oximetry leads to better postoperative cognitive performance: a pilot study. Int J Surg 2015;16:23-30. [Crossref] [PubMed]
- Fugate JE. Anoxic-ischemic brian injury. Neurol Clin 2017;35:601-11. [Crossref] [PubMed]
- Shahi V, Fugate JE, Kallmes DF, et al. Early basal ganglia hyperperfusion in acute ischemic stroke: a marker of irreversible damage? AJNR Am J Neuroradiol 2014;35:1688-92. [Crossref] [PubMed]
- Nielsen HB. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol 2014;5:93. [Crossref] [PubMed]
- Davies DJ, Su Z, Clancy MT, et al. Near-infrared spectroscopy in the monitoring of adult traumatic brain injury: A review. J Neurotrauma 2015;32:933-41. [Crossref] [PubMed]
- Moreau F, Yan R, Nambiar V, et al. Near-infrared measurements of brain oxygenation in stroke. Neurophotonics 2016;3:031403 [Crossref] [PubMed]
- Wainwright MS. Neurologic complications in the pediatric intensive care unit. Continuum (Minneap Minn) 2018;24:288-99. [Crossref] [PubMed]
- Sutter R, Tisljar K, Marsch S. Acute neurologic complications during extracorporeal membrane oxygenation: A systematic review. Crit Care Med 2018;46:1506-13. [Crossref] [PubMed]
Cite this article as: Liem S, Cavarocchi N, Hirose H. Near-infrared spectroscopy predicts brain injury in patients on extracorporeal membrane oxygenation. AME Med J 2019;4:5.