
Portal vein thrombosis: a baffling problem in cirrhosis
Portal vein thrombosis (PVT) is a vexing complication in patients with cirrhosis, often discovered during routine imaging or upon workup of a decompensating hepatic event (1). The burden of non-malignant PVT remains unclear with conflicting data on its survival impact in cirrhotics (2). Much of the data on PVT is acquired from retrospective, single center studies (3), although large datasets have been used to examine the impact of PVT in subset of populations such as liver transplant waitlist registrants and recipients (3,4). Large databases remain critical for answering large-scale epidemiologic studies, but caution is warranted when utilizing data as it needs to be interpreted within the context of the database design to avoid pitfalls and inadequate conclusions (5,6).
Sponsored by the Agency for Healthcare Research and Quality (AHRQ), the National Inpatient Sample (NIS) represents a rich source of data on administrative and demographic data from 20% of inpatient admissions with data accumulated annually since 1998, with a recent overhaul in 2012 in the sampling approach. Data elements reported include primary and secondary diagnoses, primary and secondary procedures, admission and discharge status, provider and hospital characteristics, as well as cost, length of stay, insurance status, and inpatient mortality (7).
Due to the complexity of the dataset, changes in sample design, and desire to ensure adequate interpretation of the data, AHRQ created an extensive compendium with online videos, tutorials, and documentation regarding study design, data elements, as well as best analytic strategies when using the dataset. A checklist of appropriate considerations (8) based on a recent analysis describing suboptimal adherence to published recommendations (6) accompanies the documentation (8) and iterations have been proposed elsewhere (5). AHRQ advises researchers to consider salient features such as scenarios in which estimates of incidence or prevalence can be inferred (9) and emphasizes appropriate trend analytic approaches given the 2012 sampling and weighting redesign (10).
In the present study, Cool et al. (11) attempt to ascertain prevalence of PVT in cirrhotics, in addition to clinical outcomes based on the presence of PVT. Using the NIS database from 1998–2014, they identify hospitalized patients with decompensated cirrhosis and conclude (I) PVT prevalence increased from 0.7% to 2.4%, while PVT-related inpatient mortality fluctuated with an overall decline in the study period, and (II) the presence of PVT is associated with increased risk of acute kidney injury, hepatorenal syndrome and hospital mortality (11). The association of PVT to an increased risk of acute kidney injury in hospitalized cirrhotics is not well documented in the literature, but is not a surprise as these are critically ill patients in whom any injury can have devastating consequences. The question left to clinicians is- How relevant are all these results in the context of the study design?
Figure 1 is a clinical decision tree adapted from AHRQ recommendations regarding prevalence determinations utilizing the NIS dataset (9). While the question of PVT prevalence is certainly important, the NIS does not represent an adequate source from which to gather this information, as many cases of PVT in cirrhotics do not result in inpatient admission. Thus, the study does not accurately identify the population that is most clinically important—cirrhotics with PVT. Similarly, many cases of decompensating hepatic events using the algorithm specified are managed in the outpatient clinic setting (e.g., mild cases of portosystemic encephalopathy treated with increased lactulose or ascites with diuretic titration). The rising prevalence of PVT, as well as the perceived-associated mortality of PVT are not unexpected in this hospitalized population. One strategy to ascertain the true prevalence of PVT in cirrhotics would be to use linked outpatient and inpatient records documenting presence of PVT from which associated clinical outcomes, complications, and resource utilization can be gathered.
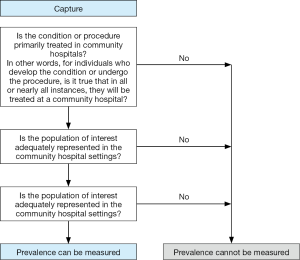
Additionally, it remains unclear which weights the authors used when analyzing trends in the years prior to 2012, as trend weights are required to be used for the period of data from 1998–2011 to account for shifts in sampling approach, and discharge weights are required for 2012 and after (10). This study attempts to examine a problem that is not well documented, as most of the recent literature describing PVT in cirrhotics focuses on peri-transplant outcomes and mortality, and large-scale data about the impact PVT has on cirrhotics outside the arena of transplantation is lacking. Nevertheless, this study echoes many previous reports regarding increased morbidity in hospitalized decompensated cirrhotics. However given the concerns regarding methodology and analysis of the study, the findings must be viewed with critical appraisal.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.01.08). The authors have no conflicts of interest declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol 2012;57:203-12. [Crossref] [PubMed]
- Chen H, Turon F, Hernandez-Gea V, et al. Nontumoral portal vein thrombosis in patients awaiting liver transplantation. Liver Transpl 2016;22:352-65. [Crossref] [PubMed]
- Englesbe MJ, Schaubel DE, Cai S, et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010;16:999-1005. [Crossref] [PubMed]
- Agbim U, Jiang Y, Kedia SK, et al. Impact of Nonmalignant Portal Vein Thrombosis in Transplant Recipients With Nonalcoholic Steatohepatitis. Liver Transpl 2019;25:68-78. [Crossref] [PubMed]
- Khera R, Krumholz HM. With Great Power Comes Great Responsibility: Big Data Research From the National Inpatient Sample. Circ Cardiovasc Qual Outcomes 2017;10. [PubMed]
- Khera R, Angraal S, Couch T, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA 2017;318:2011-8. [Crossref] [PubMed]
- HCUP Databases. Healthcare Cost and Utilization Project (HCUP). August 2018. Agency for Healthcare Research and Quality, Rockville, MD. Agency for Healthcare Research and Quality, Rockville, MD. Available online: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed December 29, 2018.
- Checklist for Working with the NIS. Healthcare Cost and Utilization Project (HCUP). November 2017. Agency for Healthcare Research and Quality, Rockville, MD. Agency for Healthcare Research and Quality, Rockville, MD. Available online: www.hcup-us.ahrq.gov/db/nation/nis/nischecklist.jsp. Accessed December 29, 2018.
- Fingar KR, Owens PL, Barrett ML, et al. Using the HCUP Databases to Study Incidence and Prevalence. HCUP Methods Series Report # 2016-06 ONLINE. December 6, 2016. U.S. Agency for Healthcare Research and Quality. Available online: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp
- HCUP NIS Trend Weights. Healthcare Cost and Utilization Project (HCUP). May 2015. Agency for Healthcare Research and Quality, Rockville, MD. Available online: www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Accessed December 29, 2018.
- Cool J, Rosenblatt R, Kumar S, et al. Portal vein thrombosis prevalence and associated mortality in cirrhosis in a nationally representative inpatient cohort. J Gastroenterol Hepatol 2018; [Crossref] [PubMed]
Cite this article as: Agbim U, Satapathy SK. Portal vein thrombosis: a baffling problem in cirrhosis. AME Med J 2019;4:11.

