
Sub-optimal diagnosis of neurogenic bladder among general practitioners in the United Kingdom—evidence from the Clinical Practice Research Datalink
Introduction
Disturbance to the normal functioning of the urinary system as a result of central nervous system (CNS) related disorders such as Parkinson’s disease (PD), multiple sclerosis (MS), spinal cord injuries (SCI) and stroke is known as neurogenic bladder (NGB) (1). Urological dysfunction manifests in different ways, ranging from retention symptoms to incontinence and sustained bladder pressures, depending on the site of neurological lesion (2). Symptoms and severity are chronic and disabling but tend to depend on the extent of the underlying neurological disease (3).
The multi-faceted and disabling nature of this condition has far-reaching effects. The symptoms of NGB and associated detrimental sequela including chronic urinary tract infection (UTI), urolithiasis and hydronephrosis poses an economic burden across the healthcare sector and has a significant impact on health-related quality of life (HRQoL) (3-5).
A lack of diagnosis (diagnosis error), is defined as ‘the failure to (a) establish an accurate and timely explanation of the patient’s health problem(s) or (b) communicate that explanation to the patient’ (6). A lack of a clear diagnosis for patients adds ambiguity to their characterisation, treatment pathways and impedes meaningful research. The most detrimental outcome of poorly managed NGB is renal dysfunction (7). A correct diagnosis means patients are more likely to have access to appropriate services and the right medical treatments, which subsequently improves their chances of optimal health outcomes and reducing costs. This review investigates the diagnosis rates of NGB in the UK using the Clinical Practice Research Datalink (CPRD) database.
Methods
The Read clinical classification is the standard medical terminology used in primary care practice in the UK. The system consists of alphanumeric codes encompassing all aspects of patient care such as clinical signs, symptoms and observations; laboratory tests; diagnoses; diagnostic and procedures performed (8).
We utilised the CPRD database to determine the number of Read coded NGB patients in the UK between 2004 and 2015 (this was a preliminary feasibility count conducted as part of a larger study, ISAC protocol number 17_027). The CPRD is the largest primary care longitudinal database containing collated anonymised patient data of over 11.3 million patients from 674 practices since 1987 (9). It is therefore largely representative of the UK population.
Keywords relating to NGB were inputted into the CPRD code browser, using the clinical, test and referral dictionaries to identify relevant Read codes. Table 1 shows the key terms that were used and the resulting Read codes that were retrieved. A medical expert confirmed all key terms and Read codes. The number of patients identified using each Read code was recorded.
Table 1
| Disease | Keywords | Read terms | Read codes |
|---|---|---|---|
| Neurogenic bladder | Neurogenic | Neurogenic bladder | K16V011 |
| Neurogenic bladder | F246112 | ||
| Neuropathic bladder | Neuropathic bladder | K16V00 | |
| Neuropathic bladder | F246113 | ||
| Reflex neuropathic bladder, NEC | K16W.00 | ||
| Uninhibited neuropathic bladder, NEC | K16X.00 | ||
| Neuromuscular bladder | Other neuromuscular dysfunctions of bladder | Kyu5200 | |
| Neuromuscular dysfunction of bladder, unspecified | Kyu5E00 |
NEC, not elsewhere classified.
Results
A total of 967 patients with a diagnosis of NGB were retrieved from the CPRD database between the 1st January 2004 and 31st December 2014. Table 2 shows the number of patients with a Read code of NGB or neuropathic bladder. No patients were found with Read codes of neuromuscular bladder, which is likely due it being replaced by newer terms.
Table 2
| Year of diagnosis | Neurogenic bladder | Neuropathic bladder | Neurogenic bladder or Neuropathic bladder |
|---|---|---|---|
| 2004 | 35 | 82 | 117 |
| 2005 | 32 | 95 | 127 |
| 2006 | 38 | 78 | 114 |
| 2007 | 29 | 68 | 96 |
| 2008 | 29 | 82 | 110 |
| 2009 | 28 | 71 | 98 |
| 2010 | 34 | 62 | 95 |
| 2011 | 41 | 56 | 97 |
| 2012 | 27 | 42 | 68 |
| 2013 | 28 | 42 | 70 |
| 2014 | 14 | 37 | 51 |
| 2015 | 10 | 8 | 18 |
| Total | 327 | 660 | 967 |
Discussion
Prevalence and incidence rates of NGB are very scarce. The only real large-scale epidemiological study was conducted using a US claims database between 2002–2007. The researchers identified 46,271 patients with NGB, however some subjects were included into the study via a proxy means of identification [overactive bladder (OAB) diagnosis or prescription of an OAB drug plus a diagnosis of a neurological condition] (10).
In the UK, 126,893 individuals were diagnosed with PD in 2009 and estimates suggest that 27–63.9% of this population experience bladder dysfunction (11,12). By conducting a very crude estimate, at the least there were 34,261 individuals with NGB secondary to PD in 2009. This is just one segment of the broader NGB population, because of course there are numerous neurological disorders that can cause NGB. Moreover, a study using the General Practice Research Database (GPRD) also identified a low frequency of NGB patients (69 patients between the years of 1987 to 2004), further compounding the suspicion that there could be an intrinsic problem in the diagnosis of NGB patients in the UK (13). This suggests that the 967 patients NGB patients retrieved from the CPRD is low. Figure 1 shows some possible speculative reasons for low diagnosis. These reasons are explored in more detail below.
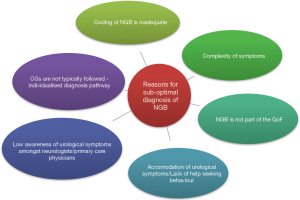
Coding of NGB
There are a multitude of different reasons for missing medical codes, and the absence of a Read code should not always be interpreted as absence of the disease itself (14).
The existence of multiple medical terminologies can make sharing and aggregating clinical information meaningfully across different levels of the healthcare sector challenging. The Read classification has been developed from a general practitioner (GP) perspective, and thus has been notoriously difficult to apply in secondary care (15). This is likely because activities and organisational structures differ between primary and secondary care, and consequently specialists and consultants have differing views to primary care healthcare professionals (HCPs) on the nature of healthcare. Some opinion suggests that ‘Read codes have failed time after time in secondary care’ (15). As a result of this ineffectuality, even if a urologist has diagnosed a patient with NGB in secondary care, the information may not be Read coded.
NGB and the Quality Outcomes Framework (QoF)
The QoF was set up in 2004 as a pay-for-performance (P4P) scheme, linking financial incentives to the quality of care (16). The scheme focused on ten key chronic conditions managed mainly in primary care including chronic heart disease and diabetes. Completeness for many of the data points in these conditions improved in the years subsequent to introduction (17).
The QoF does not include common neurological conditions such as PD, MS or SCI, nor does it include NGB. A study found that improvements related to the QoF came at the cost of small deleterious effects to conditions not incentivised under the scheme (16).
Low awareness of urological symptoms amongst non-urologists
The extensive second organ effects in neurological conditions renders a simple one-to-one physician-patient relationship insufficient to manage symptoms. In order to improve the overall QoL of MS and PD patients, The National Institute of Health and Care Excellence (NICE) recommend their needs are met through a multidisciplinary team of HCPs, including GPs, dieticians, neurologists, and psychologists, amongst others (18,19). The composition of the care team depends on the patient’s symptomology, disease severity and progression, as well as their social and psychological wellbeing.
Their superior expertise in bladder dysfunction positions urologists as pre-eminent in the diagnosis and management of NGB, however, they are only included in the multidisciplinary team, based on their perceived necessity. For example, if urological symptoms are not severe, conservative management techniques such as the administration of OAB drugs and introducing patients to catheterisation is easily performed in primary care.
Although resources are saved by confining management to primary care, it runs the risk of NGB patients remaining undiagnosed because awareness of urological symptoms amongst GPs is notoriously low. A report into continence care in the UK found that GPs do not routinely query ‘at risk’ individuals about their continence issues (20). Some of the common reasons include the fear of being unable to match patient expectations, a lack of understanding of urological symptoms and a lack of confidence in treating OAB (21).
Assigning a diagnosis is not a straightforward task, often proving challenging, especially in primary care. This particularly holds true in NGB, where symptomology can differ vastly between patients, making it difficult to uniformly apply diagnostic recommendations from clinical guidelines. Furthermore, there is a large degree of symptom overlap with idiopathic OAB, which can make distinguishing these conditions difficult for the untrained professional. Therefore, patients could be incorrectly diagnosed with OAB rather than NGB. Given the diffuse and often severer nature of NGB, it is important that the distinction between these conditions is made.
In most areas of the UK, neurological specialist nurses play an instrumental role in streamlining care from multiple providers to create an individualised management pathway (22,23). Although multidisciplinary care for neurological patients is crucial, communication amongst HCPs can often prove suboptimal, and is further exacerbated by the fragmented healthcare service (24,25). This ultimately impedes access to urological services, and hence receiving a diagnosis of NGB on time. Such a scenario is particularly likely in the current climate of austerity, where the number of nurse specialists working within the community are declining (26). Patients may therefore have to rely on their GP, who, as established have less awareness of urological symptoms and therefore are less likely to be able to diagnose NGB or refer patients to a urologist.
Another possible rationalisation for low diagnosis rates could pertain to the attending HCPs decision to focus on the primary neurological pathology, since managing it usually improves the symptoms of NGB. Therefore, although symptoms may be adequately managed, patients may not receive a formal diagnosis from a urologist.
Accommodation of urological symptoms
Patients with neurological disorders experience life-altering symptoms such as loss of mobility, problems with coordination, memory loss and severe pain (27). In contrast to their incapacitating symptoms, patients may not view their urological dysfunction as severe (i.e., an accommodation of symptoms occurs), which can result in a lack of help seeking behaviour (3). Other reasons for avoiding HCP contact include; embarrassment around OAB symptoms, lack of faith in treatment and self-management of symptoms (28). If patients do not reveal their symptoms, they cannot receive a diagnosis and consequently, treatment for their condition.
Implications of low diagnosis and potential solutions
For optimal management in NGB, closing the diagnosis gap is essential. Deprived of a diagnosis, patients will face an uphill battle in gaining access to services and appropriate medications. This increases the chances of unpredicted situations, secondary conditions and hospitalisations, placing an additional strain on the National Healthcare Service (NHS) (29). It is already known that a number of serious sequela complicate the management of NGB, as well as evidence to suggest a substantial cost to the healthcare system (3). The issue of health inequality also arises, as those primarily affected will be in areas of the UK experiencing severe underfunding and cuts in specialist nurses; the key facilitators of the NGB care pathway.
The issue of interoperability between primary and secondary care could be solved through the gradual migration underway in UK clinical practice from Read codes to the Systematised Nomenclature of Medicine Clinical Terms (SNOMED CT) (15). It is described as the ‘most comprehensive and precise’ CT in the world (30). It is envisioned that implementation of SNOMED CT in UK clinical practice will improve the channel of communication between primary and secondary care (15). It will be of value to assess whether the diagnosis of NGB improves after implementation of SNOMED CT is complete.
Considering the high prevalence of bladder symptoms in patients with neurological conditions the permanent inclusion of a urologist in the multidisciplinary team would be a positive move towards improved diagnosis rates in NGB. Furthermore, effort towards enhancing the visibility of disease through national awareness campaigns targeting GPs, patients and carers could further improve the diagnosis rates. In particular, campaigns highlighting that urological symptoms emanating from neurological conditions are very common, would be instrumental in changing perceptions and attitudes amongst these stakeholders.
Lessons can be learnt from the multiple successful campaigns carried out in the field of idiopathic OAB. One example is the campaign launched by the American Urological Association (AUA) entitled ‘It’s Time to Talk About OAB’, which aimed to alleviate the stigma surrounding talking to a physician about OAB symptoms and equip patients with a better understanding of their condition. The campaign consisted of a website featuring patient education materials and a ‘Voices of OAB’ contest, where patients shared testimonials of life with OAB (31).
Financial incentives can improve reporting and coding, as evidenced by the QoF scheme. The NHS could offer financial incentives to GPs for referrals to a urologist, who are experienced in identifying NGB and differentiating it from idiopathic OAB. Health economic analysis into the cost-effectiveness of encouraging referrals over management in primary care would be necessary to ensure the efficacy of introducing such a measure.
Clinical decision support systems (CDSS) represent a sophisticated computational means by which clinical guidelines can be integrated into clinical practice, assisting GPs with any diagnostic uncertainty surrounding NGB (32). Some recent systematic reviews of trials of CDSS have demonstrated promising results, however some conflicting evidence concludes there is a lack of data demonstrating benefit for patient outcomes (32,33). In any case, the use of information technology (IT) alone is not sufficient. Better relationships amongst stakeholders are imperative to improve the diagnosis and referral rates in NGB; this includes improving doctor-patient relationships, so patients feel comfortable sharing their symptoms with their doctor. Additionally, strengthening the channels of communication between doctors and specialists is fundamental in facilitating information exchange and creating learning opportunities for GPs to enhance their understanding of NGB.
Limitations
This is by no means an exhaustive analysis of the potential reasons for low NGB diagnosis rates. This paper did not explore possible shortcomings in current diagnostic practices. Furthermore, it is important to consider that the reasons presented are purely speculative, and the determinants of referrals should be understood through other means. The CPRD database could be used to conduct correlation studies against factors such as socio-economics, sex, and comorbidity (34,35). Simulated patients described by case vignettes could be used to in future studies to measure variation in clinicians’ approaches to diagnosis and treatment (36).
Conclusions
Improving the diagnosis rates of NGB in the UK will allow proper provision of care and services. Measures such as improved interoperability between databases, educational campaigns, financial incentives, CDSS and fostering better relationships between important stakeholders are instrumental. Implementing these measures will enhance patient characterisation, help devise better management strategies, facilitate efficient resource allocation and ultimately improve health outcomes for NGB patients. The authors of this paper suggest hypothesis testing studies to ascertain the actual determinants of referrals.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.02.02). A Jaggi worked full-time at Astellas Pharma EU under a Knowledge Transfer Partnership (KTP) with Manchester Metropolitan University (MMU) during the time of the analysis of this study. F Fatoye has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the declaration of Helsinki (as revised in 2013). The institutional ethical review and informed consent were waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Game X, Fowler CJ, Panicker J. Neuropathic Bladder Dysfunction. Trends in Urol, Gynae & Sexual Health 2010;15:23-8.
- Haab F. Chapter 1: The Conditions of Neurogenic Detrusor Overactivity and Overactive Bladder. Neurourol Urodyn 2014;33:S2-5. [Crossref] [PubMed]
- Tapia CI, Khalaf K, Berenson K, et al. Health-related quality of life and economic impact of urinary incontinence due to detrusor overactivity associated with a neurologic condition: a systematic review. Health Qual Life Outcomes 2013;11:13. [Crossref] [PubMed]
- Davis A, Turner B, Ramadhan M, et al. The burden of bladder dysfunction in multiple sclerosis: A multi-centre audit. J Neurol Neurosurg Psychiatry 2016;87:e1.32-e1.
- Flack C, Powell CR. The Worldwide Economic Impact of Neurogenic Bladder. Curr Bladder Dysfunct Rep 2015;10:350-4. [Crossref] [PubMed]
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine, et al. Improving Diagnosis in Health Care. Balogh EP, Miller BT, Ball JR. editors. Washington (DC): National Academies Press, 2015.
- Nseyo U, Santiago-Lastra, Y. Long-Term Complications of the Neurogenic Bladder. In: Stoffel JT, Santiago-Lastra Y, Taneja SS. editors. The Impact of Neurologic Disease on the Urinary Tract. Philadelphia, US: Elsevier, 2017:361.
- Herrett E, Thomas SL, Schoonen WM, et al. Validation and Validity of Diagnoses in the General Practice Research Database: a Systematic Review. Br J Clin Pharmacol 2010;69:4-14. [Crossref] [PubMed]
- Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. [Crossref] [PubMed]
- Manack A, Motsko SP, Haag-Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn 2011;30:395-401. [Crossref] [PubMed]
- Parkinson’s prevalence in the United Kingdom. 2009. Available online: https://www.parkinsons.org.uk/sites/default/files/parkinsonsprevalenceuk_0.pdf. Accessed 1st Dec 2017.
- Ruffion A, Castro-Diaz D, Patel H, et al. Systematic Review of the Epidemiology of Urinary Incontinence and Detrusor Overactivity among Patients with Neurogenic Overactive Bladder. Neuroepidemiology 2013;41:146-55. [Crossref] [PubMed]
- Odeyemi IA, Dakin HA, O'Donnell RA, et al. Epidemiology, prescribing patterns and resource use associated with overactive bladder in UK primary care. Int J Clin Pract 2006;60:949-58. [Crossref] [PubMed]
- Lo Vecchio A, Giannattasio A, Duggan C, et al. Evaluation of the quality of guidelines for acute gastroenteritis in children with the AGREE instrument. J Pediatr Gastroenterol Nutr 2011;52:183-9. [Crossref] [PubMed]
- Meek T. SNOMED to replace Read codes by 2020. 2015. Available online: https://www.digitalhealth.net/2015/10/snomed-to-replace-read-codes-by-2020/. Accessed 11th Sept 2018.
- Doran T, Fullwood C, Kontopantelis E, et al. Effect of financial incentives on inequalities in the delivery of primary clinical care in England: analysis of clinical activity indicators for the quality and outcomes framework. Lancet 2008;372:728-36. [Crossref] [PubMed]
- Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of Chronic Obstructive Pulmonary Disease Recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open 2014;4:e005540 [Crossref] [PubMed]
- NICE. Multiple sclerosis in adults: management. In: Clinical Guideline [CG186]. 2014. Available online: https://www.nice.org.uk/guidance/cg186/chapter/1-recommendations. Accessed 19th Sept 2018.
- NICE. Parkinson's Disease. In: Quality Standard [QS164]. UK. 2018. Available online: https://www.nice.org.uk/guidance/qs164/chapter/Quality-statement-1-Point-of-contact-with-specialist-services. Accessed 19th Sept 2018.
- Wagg A, Harari D, Husk J, Lowe D, et al. National Audit of Continence Care. London: Royal College of Physicians, 2010.
- Smith AL, Nissim HA, Le TX, et al. Misconceptions and miscommunication among aging women with overactive bladder symptoms. Urology 2011;77:55-9. [Crossref] [PubMed]
- Bhidayasiri R, Boonpang K, Jitkritsadakul O, et al. Understanding the role of the Parkinson's disease nurse specialist in the delivery of apomorphine therapy. Parkinsonism Relat Disord 2016;33:S49-55. [Crossref] [PubMed]
- MacMahon DG. Parkinson's disease nurse specialists: an important role in disease management. Neurology 1999;52:S21-5. [Crossref] [PubMed]
- NICE. Urinary incontinence in neurological disease: management of lower urinary tract dysfunction in neurological disease: National Institute for Health and Clinical Excellence, 2012.
- Gallacher KI, Batty GD, McLean G, et al. Stroke, multimorbidity and polypharmacy in a nationally representative sample of 1,424,378 patients in Scotland: implications for treatment burden. BMC Med 2014;12:151. [Crossref] [PubMed]
- Christodoulou M. Neurological nurse specialists: a vital resource under threat. Lancet Neurol 2012;11:210-1. [Crossref] [PubMed]
- Guy L, R. Brain Disorders. 2017. Available online: https://www.healthline.com/health/brain-disorders. Accessed 19th July 2018.
- Diokno AC, Sand PK, Macdiarmid S, et al. Perceptions and behaviours of women with bladder control problems. Fam Pract 2006;23:568-77. [Crossref] [PubMed]
- Vize R. Reality of the NHS budget squeeze. BMJ 2011;343:d8027. [PubMed]
- SNOMED. SNOMED CT - The global language of healthcare. 2018. Available online: https://www.snomed.org/snomed-ct. Accessed 18th Sept 2018.
- AUA. AUA Foundation Launches Campaign to Address Stigma of Overactive Bladder. 2012. Available online: https://www.prnewswire.com/news-releases/aua-foundation-launches-campaign-to-address-stigma-of-overactive-bladder-159573595.html. Accessed 1st May 2018.
- Hemens BJ, Holbrook A, Tonkin M, et al. Computerized clinical decision support systems for drug prescribing and management: A decision-maker-researcher partnership systematic review. Implement Sci 2011;6:89. [Crossref] [PubMed]
- Fillmore CL, Bray BE, Kawamoto K. Systematic review of clinical decision support interventions with potential for inpatient cost reduction. BMC Med Inform Decis Mak 2013;13:135. [Crossref] [PubMed]
- Chan BT, Austin PC. Patient, physician, and community factors affecting referrals to specialists in Ontario, Canada: a population-based, multi-level modelling approach. Med Care 2003;41:500-11. [Crossref] [PubMed]
- Zielinski A, Borgquist L, Halling A. Distance to hospital and socioeconomic status influence secondary health care use. Scand J Prim Health Care 2013;31:83-8. [Crossref] [PubMed]
- Bachmann LM, Mühleisen A, Bock A, et al. Vignette studies of medical choice and judgement to study caregivers' medical decision behaviour: systematic review. BMC Med Res Methodol 2008;8:50. [Crossref] [PubMed]
Cite this article as: Jaggi A, Fatoye F. Sub-optimal diagnosis of neurogenic bladder among general practitioners in the United Kingdom—evidence from the Clinical Practice Research Datalink. AME Med J 2019;4:16.

