
Long-term follow-up and treatment in a case of PAFIYAMA syndrome: urgent need for identifying related biomarkers
Introduction
An increasing number of healthy and young or middle-aged recreational, amateur and professional athletes (cyclists, runners, rowers, cross-country skiers, among others) call for medical attention complaining about episodes of symptomatic palpitations which, in many cases, definitely correspond to episodes of paroxysmal atrial fibrillation (AF). It is now well-established that long-term strenuous exercise increases the risk of new-onset AF (1,2). In this regard, based on the evidence accumulated in the literature, the paroxysmal atrial fibrillation in young and middle-aged athletes (PAFIYAMA) syndrome has been recently described as a new entity with specific characteristics (3,4). Due to its particular pathogenesis, the clinical management of this syndrome has not been clearly described so far (5). In order to increase the knowledge of the clinical management of this entity, we present here a follow-up treatment of a PAFIYAMA syndrome case in which a catheter ablation of the pulmonary veins (PVs) was finally required to successfully terminate the AF episodes.
Case presentation
A 42-year-old man was admitted to the Emergency Department (ED) complaining about poorly tolerated, rapid and irregular palpitations. A characteristic pattern of AF was observed in a 12-lead electrocardiogram (ECG) (Figure 1). The patient reported that the palpitations started after participating in a half-marathon, although he also noticed several self-limited episodes of rapid and irregular palpitations (less than 5 min of duration) during the last months (predominantly nocturnal and/or after meals). The patient denied toxic habits or illicit drug use. Blood and urine tests were within the normal ranges. He is a long-term endurance athlete (runner and rower) who started exercising in his 20 s (>12 h/week with intensity greater than 60% of maximum heart rate). In ED, the patient was administered with enoxaparin (60 mg/SC) and amiodarone (300 mg/IV), slowing the heart rate and restoring the normal sinus rhythm. Since the CHA2DS2-VASc score was 0, oral anticoagulation was not initiated. Afterwards, the patient was discharged and transferred to the cardiology unit for additional evaluations and follow-ups.
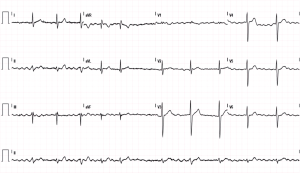
While waiting to be evaluated by the cardiologist, the patient had a poorly tolerated relapse of AF and he was again admitted to the ED. The transesophageal echocardiography confirmed the absence of mobile left atrial (LA) thrombus. Thereafter, the patient underwent electrical cardioversion (biphasic DC shock, 150 J) and sinus rhythm was restored. The echocardiography showed a concentric left ventricle (LV) hypertrophy: inter-ventricular septal thickness in diastole (IVSd): 14 mm, and no alterations in contractility. A moderate LA dilation (42 mm, 20.5 cm2) with a normal left ventricle ejection fraction (LVEF) and normal diastolic function was also observed. The patient was then discharged with oral medication as follows: flecainide 100 mg/12 h, bisoprolol 10 mg/d and apixaban 5 mg/12 h. Six days after the first electrical cardioversion, the patient presented recurrence of AF. After one month of pharmacological treatment, the patient underwent electrical cardioversion once again (three biphasic DC shocks, 150, 200 and 200 J). On this occasion, the cardioversion was ineffective and the patient remained in AF. Oral flecainide was suspended due to severe bradycardia, but bisoprolol and apixaban were kept.
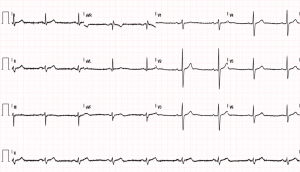
The patient was transferred to the Arrhythmia and Electrophysiology Unit (Hospital Universitario Clinico Valencia, Spain). A resting cardiac magnetic resonance imaging (MRI) was performed, showing a septal hypertrophy with an interventricular septal thickness of 14 mm, normal volumes and LVEF (69%), and normal drainage of all four PVs. Given the functional limitation of the patient and his inability to exercise, the specialists recommended he undergo a circumferential cryoablation of the PVs, which the patient agreed to. An echocardiogram was performed immediately after ablation and showed no complications, whilst the patient remained in sinus rhythm (Figure 2). A Holter-ECG performed three months after the cryoablation of the PVs showed no signs of arrhythmia. The patient has resumed light-to-moderate intensity physical exercise with no cardiac rhythm alterations.
Discussion
We present here a patient fulfilling the PAFIYAMA syndrome criteria (3). Athletes in AF have relatively slow ventricular rates, low CHADSVASc scores as well as fewer symptoms and a better quality of life than the general population with AF. Athletes with AF should be advised to reduce their exercise intensity and duration (6-8). Accordingly, discontinuing training for 2 months to stabilize sinus rhythm is recommended in these patients (3), despite their usual refusal to cease exercise or even reduce its intensity and/or loads (6). However, this strategy is not useful in all cases, particularly when AF episodes are poorly tolerated (6). Likewise, the pill in pocket approach seems to be an effective and elective option until we have more data about PAFIYAMA syndrome as well as how to prevent it (9), whereas PVs ablation should be considered a last alternative. In effect, the CABANA trial showed that ablation is not superior to drug therapy for cardiovascular outcomes at 5 years among patients with new-onset or untreated AF that required therapy (10). Nonetheless, as happened in this patient, antiarrhythmic therapy is not tolerated well because of sinus bradycardia and other adverse effects. Ablation of PVs appears to be an option in patients with not well-tolerated PAFIYAMA syndrome who are determined to continue exercising.
Last but not least, numerous potential mechanisms such as exercise-associated inflammation and fibrosis have been proposed to explain the link between strenuous exercise and new-onset AF (9,11,12). Interestingly, provided this syndrome is finally confirmed, identifying specific biomarkers is urgently needed in order to monitoring and predicting the risk of cardiac maladaptation in response to exercise, especially in the atria, and thus prevent PAFIYAMA syndrome (9).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mont L, Tamborero D, Elosua R, et al. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace 2008;10:15-20. [Crossref] [PubMed]
- Brunetti ND, Santoro F, Correale M, et al. Incidence of atrial fibrillation is associated with age and gender in subjects practicing physical exercise: A meta-analysis and meta-regression analysis. Int J Cardiol 2016;221:1056-60. [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Lippi G, et al. Atrial fibrillation in highly trained endurance athletes - Description of a syndrome. Int J Cardiol 2017;226:11-20. [Crossref] [PubMed]
- Cervellin G, Sanchis-Gomar F, Filice I, et al. Paroxysmal atrial fibrillation in young and middle-aged athletes (PAFIYAMA) syndrome in the real world: a paradigmatic case report. Cardiovasc Diagn Ther 2018;8:176-9. [Crossref] [PubMed]
- Elliott AD, Mahajan R, Lau DH, et al. Atrial Fibrillation in Endurance Athletes: From Mechanism to Management. Cardiol Clin 2016;34:567-78. [Crossref] [PubMed]
- Perez-Quilis C, Lippi G, Cervellin G, et al. Exercising recommendations for paroxysmal AF in young and middle-aged athletes (PAFIYAMA) syndrome. Ann Transl Med 2017;5:24. [Crossref] [PubMed]
- Furlanello F, Bertoldi A, Dallago M, et al. Atrial fibrillation in elite athletes. J Cardiovasc Electrophysiol 1998;9:S63-8. [PubMed]
- Hoogsteen J, Schep G, Van Hemel NM, et al. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace 2004;6:222-8. [Crossref] [PubMed]
- Perez-Quilis C, Lippi G, Mena S, et al. PAFIYAMA syndrome: prevention is better than cure. J Lab Precis Med 2016;1:8. [Crossref]
- Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1261-74. [Crossref] [PubMed]
- Sanchis-Gomar F, Lucia A. Pathophysiology of atrial fibrillation in endurance athletes: an overview of recent findings. CMAJ 2016;188:E433-5. [Crossref] [PubMed]
- Brunetti ND, Tarantino N, Santoro F, et al. "PAFIYAMA" syndrome; further evidence on a novel clinical entity. Int J Cardiol 2018;256:9. [Crossref] [PubMed]
Cite this article as: de la Guía Galipienso F, Valle-Muñoz A, Alania Torres E, Meyer-Josten C, Sanchis-Gomar F. Long-term follow-up and treatment in a case of PAFIYAMA syndrome: urgent need for identifying related biomarkers. AME Med J 2019;4:34.

