Empty hemiscrotum and a giant abdominal mass case report
Introduction
Testicular cancer accounts for less than 1% of all male tumors, and it is the most common solid tumor in men aged 20–35 years (1,2). Testicular germ cell tumors (TGCTs) account for 95% of malignant tumors arising from the testes (2-4). The risk factors for testis cancer include personal or family history of testicular cancer, presence of intra-tubular germ cell neoplasia (ITGCN), as well as cryptorchidism (5). The early detection of TGCTs is of paramount importance as stage I tumors confined to the testes have a survival rate of up to 100% (6). In the early stages, TGCTs most commonly present as a painless testicular nodule, whereas, in the advanced states patient can present with supraclavicular or retroperitoneal lymphadenopathy, gynecomastia, or pulmonary emboli (7). Metastatic spread of TGCTs follows the lymphatic drainage of the testes to the retroperitoneal lymph nodes, therefore, persistent retroperitoneal lymphadenopathy following chemotherapy requires surgical resection (7). Undescended testicle (UDT) is a significant risk factor for the development of TGCTs, therefore pre-pubertal orchiopexy should be strongly considered to reduce the risk and facilitate early detection of TGCTs (8). However, in this case study, we report a cryptorchid adult patient presenting with an empty hemiscrotum, and a large abdominal mass secondary to TGCT. This highlights the significance of proper genitourinary assessment and close follow up of patients with UDT.
Case presentation
A 26-year-old male presented to the emergency department due to a broken jaw following an altercation. On physical examination, he was noted to have a large intraabdominal mass measuring 13 cm, as well as an empty left hemiscrotum. Before work up of this mass could be initiated, the patient was lost to follow up.
One year later, the patient re-presented, with the intraabdominal mass now measuring 17 cm. He also has an elevated alpha-fetoprotein (AFP) of 159 ng/mL. He received two cycles of bleomycin, etoposide, and cisplatin for a presumed mixed non-seminomatous germ cell tumor of his undescended left testicle. He was then lost to follow up again.
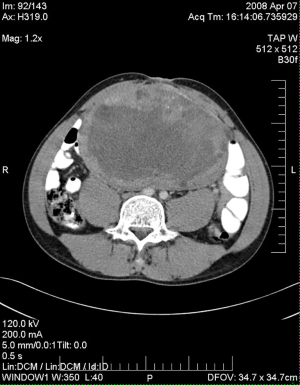
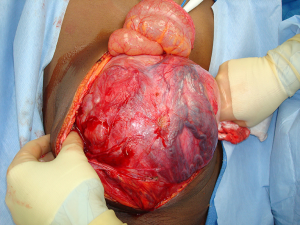
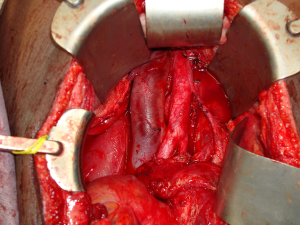
The patient presented a third time, another year later. At that time, the mass in the UDT measured 19-cm (Figure 1). Serum tumor markers included an AFP of 5,310 ng/mL, beta-human chorionic gonadotrophin (β-hCG) of 0.5 MIU/mL, and a lactate dehydrogenase (LDH) of 846 U/L. The patient completed four cycles of paclitaxel, ifosfamide, and cisplatin. Following systemic chemotherapy, there was radiographic evidence of reduction in the size of the abdominal mass and normalization of serum tumor markers. The patient then underwent a left orchiectomy (Figure 2), bilateral full template retroperitoneal lymph node dissection, and partial cystectomy secondary to intimate involvement with the left side of the bladder (Figure 3). Final pathology revealed a malignant mixed germ cell tumor consisting of 75% immature teratoma, 25% mature teratoma, negative surgical margins, and involvement of zero of twenty-three retroperitoneal lymph nodes. The patient recovered well and thus far has no evidence of disease recurrence.
Discussion
Testicular GCTs is the most common solid malignancy in males between 20–35 years (1,2). Despite its uncommon presentation, the incidence has been on the rise (1,2). The 2018 estimates from the United States suggested diagnosis of over 9,000 new cases of testicular GCTs, resulting in approximately 400 deaths (1,2).
Testicular GCTs are categorized into seminoma or non-seminoma (9). Seminomas tend to be more common in older patients, with non-seminoma in younger patients. Non-seminomatous GCTs (NSGCT) are further sub categorized into four histological types: yolk sac, embryonal, choriocarcinoma, or teratoma (9). NSGCTs tend to have fast growth kinetics and can harbor more than one histological subtype in up to 60% of cases (9). Serum tumor markers secreted by GCTs such as AFP, ß-HCG and LDH are essential for diagnosis, prognostication, and assessment of treatment response (10).
Men with UDT have increased risk of developing TGCTs and the relative risk (RR) of developing testicular cancer is approximately 2.6-8 (11). Pre-pubertal orchidopexy tends to reduce the RR to 2.23 (11). Therefore, the American Urologic Association Guidelines recommends that physicians carefully examine the affected and the normal contralateral testis, noting their relative size and consistency (12). Any firm testicular nodules should be considered suspicious for malignancy and the patient also should be examined for evidence of palpable abdominal mass or pain, gynecomastia, and supraclavicular lymphadenopathy (12). Furthermore, the guidelines also highlight the significance of close follow up in patients with cryptorchidism and self-examination after puberty (12).
The International Germ Cell Cancer Collaborative Group (IGCCCG) developed guidelines for staging patients with metastatic NSGCTs based on the prognosis of patients following orchiectomy (13). This staging system stratifies patients into good, intermediate, or poor prognosis, in order to tailor their systemic chemotherapy (13). According to the IGCCCG, NSGCT’s with a good prognosis should meet all of the following criteria: the primary tumor should involve the testis or retroperitoneum, there should be no visceral metastasis to sites other than the lungs, the AFP levels should be below 1,000 ng/mL, ß-HCG levels should be below 5,000 IU/L, and the LDH levels should be below 1.5 times the upper limit of normal (ULN) (13). NSGCT patients with an intermediate prognosis have criteria that are the same as a patient with good prognosis, except either the AFP level is between 1,000 to 10,000 ng/mL, the β-HCG level is between 5,000 to 50,000 IU/L, or the LDH level is between 1.5 to 10× ULN (13). The NSGCT patients with poor prognosis should meet any one of the following criteria: the primary tumor is in the mediastinum, there are visceral metastasis in sites outside the lungs, the AFP level is over 10,000 ng/mL, the β-HCG level is over 50,000 IU/L, or the LDH level is over 10× ULN (13).
According to the 2019 National Comprehensive Cancer Network Guidelines, the treatment of choice for metastatic disease in NSGCT patients with a good prognosis is three cycles of the bleomycin, etoposide, and cisplatin (BEP), whereas, in the intermediate/poor prognosis group BEP is administered over four cycles (14). In patients with limited response, the second-line chemotherapeutic option is etoposide, ifosfamide, and cisplatin (VIP) (14).
Teratomas are tumors which contain well-differentiated or incompletely differentiated elements of at least two of the three germ cell layers which include endoderm, mesoderm, and ectoderm (15,16). Mature teratomas are well-differentiated, whereas, immature teratomas tumors tend to have incompletely differentiated components (15,16). However, this distinction does not affect the clinical management of adult patients (9). Mature teratomas can also harbor solid or cystic components that can be comprised of bone, cartilage, teeth, hair, and squamous epithelium (15,16).
Pure testicular teratomas account for 4% of TGCTs and approximately 47% of mixed adult GCTs harbor teratoma at their metastatic sites (15,16). Overall, testicular teratomas are associated with normal serum tumor markers, but may present with mildly elevated serum AFP (15). Despite benign histologic appearance, teratomas may contain numerous genetic abnormalities such as, aneuploidy, chromosome 12 amplification, and variable proliferative capacity (17). In some reports, the cystic fluid within the teratoma has been reported to contain β-hCG and AFP, suggesting its malignant potential (17).
The genetic instability of teratomas may lead to uncontrollable proliferation and local invasion resulting in lethal growing teratoma syndrome (18). Furthermore, on rare occasions, teratomas may transform into rhabdomyosarcoma, adenocarcinoma, or neuroectodermal tumor resulting in a highly aggressive and chemo-resistant phenotype which is associated with a poor prognosis and death (19). Interestingly, the natural history of pre-pubertal teratoma demonstrates benign behavior with low malignant potential and lower propensity for recurrence (16). Currently, published reports on intraabdominal teratomas derived from UDTs have been largely confined to case reports in the pediatric population.
In summary, we present a case of a large teratoma arising from UDT in an adult male patient. He first presented with IGCCCG good risk features and completed two cycles of BEP for presumed mixed TGCT. On his subsequent presentation, he was upstaged to IGCCCG intermediate risk category due to even higher level of AFP expression and was treated with paclitaxel, ifosfamide, and cisplatin followed by successful consolidative surgery. This case demonstrates that testicular teratomas can also arise within UDT of adult cryptorchid patients and the significance of genitourinary assessment as part of routine physical examination in boys and young adult men.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Rare Genitourinary Malignancies”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.11.02). The series “Rare Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. Dr. Spiess served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of AME Medical Journal from Sep 2017 to Feb 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- SEER Cancer Statistics Factsheets: Testis Cancer. National Cancer Institute. Bethesda, MD. 2018.
- Winter C. Testicular germ cell tumors: pathogenesis, diagnosis, and treatment. Nat Rev Endocrinol 2011;7:43-53. [Crossref] [PubMed]
- Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int 2009;104:1329-33. [Crossref] [PubMed]
- Turnbull C, Rahman N. Genome-wide association studies provide new insight into the genetic basis of testicular germ-cell tumour. Int J Androl 2011;34:e86-96; discussion e96-7.
- Dieckmann KP, Richter-Simonsen H, Kulejewski M, et al. Testicular Germ-Cell Tumours: A Descriptive Analysis of Clinical Characteristics at First Presentation. Urol Int 2018;100:409-19. [Crossref] [PubMed]
- Bahrami A, Ro JY, Ayala AG. An overview of testicular germ cell tumors. Arch Pathol Lab Med 2007;131:1267-80. [PubMed]
- Banerji JS, Singh JC. Does early orchidopexy reduce risk of testicular cancer?. Indian J Urol 2008;24:430-1. [PubMed]
- Vasdev N, Moon A, Thorpe AC. Classification, epidemiology, and therapies for testicular germ cell tumors. Int J Dev Biol 2013;57:133-9. [Crossref] [PubMed]
- Milose JC, Filson CP, Weizer AZ, et al. Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open Access J Urol 2011;4:1-8. [PubMed]
- Wood HM, Elder JS. Cryptochidism and testicular cancer: Separating fact and fiction. J Urol 2009;181:452-61. [Crossref] [PubMed]
- Kolon TF, Herdon CD, Baker LA, et al. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol 2014;192:337-45. [Crossref] [PubMed]
- International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: A Prognostic Factor-Based Staging System for Metastatic Germ Cell Cancers. J Clin Oncol 1997;15:594-603. [Crossref] [PubMed]
- NCCN Guidelines Version 1.2019. Testicular Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/testicular.pdf
- Leibovitch I, Foster RS, Ulbright TM, et al. Adult primary pure teratoma of the testis. The Indiana experience. Cancer 1995;75:2244-50. [Crossref] [PubMed]
- Simmonds PD, Lee AH, Theaker JM, et al. Primary pure teratoma of the testis. J Urol 1996;155:939-42. [Crossref] [PubMed]
- Sella A, el Naggar A, Ro JY, et al. Evidence of malignant features in histologically mature teratoma. J Urol 1991;146:1025-8. [Crossref] [PubMed]
- Logothetis CJ, Samuels ML, Trindade A, et al. The growing teratoma syndrome. Cancer 1982;50:1629-35. [Crossref] [PubMed]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising among men with germ cell tumors. J Urol 1998;159:133-8. [Crossref] [PubMed]
Cite this article as: Rudzinski JK, Carlock HR, Beech BB, Zargar-Shoshtari K, Sharma P, Kim T, Spiess PE. Empty hemiscrotum and a giant abdominal mass case report. AME Med J 2019;4:42.