
Advances in penile reconstructive techniques for primary penile tumors
Introduction
Penile squamous cell carcinoma (SCC) is a rare but critically damaging malignancy. Traditionally, the mainstays of therapy had been radical surgery with total or partial penectomy. However, this traditional strategy has often led to poor cosmetic results, difficulty in urinating while standing, and sexual dysfunction in many men. Negative impacts were subsequently reported in the quality of life for these men and their partners. The modern age has given rise to a paradigm shift in the management of this cancer, with an increased emphasis on organ-sparing approaches without compromising oncologic control (1). These approaches avoid radical surgery where possible. The field has advanced beyond arguments that these attempts to spare the penis compromise patient survival in the face of such a potentially fatal disease.
A retrospective review of 1,000 patients noted that although 27 percent of those who underwent penile-sparing surgery had local recurrence at 5 years follow up compared to almost 4 percent of those who underwent partial penectomy, there was no significant different in survival (2). Indeed, another smaller study of 63 patients found that this difference in local recurrence rates was again seen specifically for T1 tumors, with 31 percent recurrence rate for penile-sparing techniques versus 0 percent for conventional amputation at 5 year follow up, but there was no difference in overall survival. Those patients who were found to recur were able to undergo salvage local resection of residual SCC (3).
When penile amputation cannot be avoided, developments in reconstructive techniques bolster the ideal that patient quality of life is a fundamental treatment goal after curative intent by way of sparing penile tissue whenever appropriate (4). This report aims to present these recent developments to the discerning urologist looking to fine-tune an individualized approach to diagnosing and managing penile SCC. The reader will find these advances in reconstruction for penile cancer essential to the patients and their partners’ successful outcomes in navigating this potentially morbid disease.
Epidemiology of penile cancer
Penile cancer remains a rare disease with high rates of morbidity and mortality, predominantly occurring in men greater than 60 years old with an estimated 26,000 cases diagnosed annually around the globe (5). The vast majority of penile cancer, up to 93 percent is histologically SCC (6). Prognosis with penile cancer is largely affected by stage, grade and lymph node involvement at the time of diagnosis (7).
Trends in penile cancer incidence vary geographically over different populations with highest incidence in some of the developing countries (8). In Western European countries and the United States, penile cancer accounts for a small percentage of overall malignancy, with incidence ranges from 0.3 to 1.0 per 100,000, age standardized, whereas penile cancer constitutes 6–10 percent of malignancy in developing countries in Asia, Africa and South America (5,9). In a study of penile cancer incidence in the Netherlands, spanning 60 years, penile incidence increased over time (8). However, in the US and Finland, decreased incidence in penile cancer was noted (6,7,10) with rates varying by race and ethnicity. In the US studies, Goodman and others used the Surveillance, Epidemiology and End Results database to analyze trends, noting higher rates of penile cancer among Hispanic men compared to other ethnic groups. There was no difference in incidence between whites and blacks (6). The downward trend in US incidence is speculated to be due to convergence of circumcision rates among younger men of all races and ethnicities in the past few decades. Black men older than 80 years old had a significantly higher rate of penile cancer and low rates of circumcision, which was common in the 1940s and 1950s (6). In Finland, the 24-year study of differences in incidence rates of male genital cancers showed no clear association between penile cancer incidence and social class variation. Smoking, which is a consistent risk factor for penile cancer, was more prevalent among lower social classes (10).
Common known risk factors for penile cancer include increasing age, tobacco use, lack of neonatal circumcision, especially those with poor penile hygiene, phimosis, chronic inflammatory conditions including balanitis and lichen sclerosis, treatment with ultraviolet A photochemotherapy, and human papillomavirus (HPV) status, with the two most consistently reported risk factors being lack of neonatal circumcision and HPV status (11-13). A Medline review of articles outlining penile cancer risk factors, published from 1966–2000, showed a threefold decreased risk of penile cancer development in those neonatally circumcised (14). Self-reported condyloma was associated with three to five-fold increase in penile cancer. Cervical cancer in the wife was not associated with an increased risk of penile cancer in the husband (14).
Sexually transmitted human papilloma viruses are implicated in multiple malignancies including penile cancer, specifically oncogenic HPV strains 16, 18, 31 and 33. An estimated 36 to 50 percent of penile cancers are attributable, at least in part to HPV infection (15,16). In a systematic review of HPV prevalence in invasive penile cancer, over 1,200 patients were analyzed. HPV prevalence was 47.9 percent, ranging from 22.4 percent to 66.3 percent over subtypes. HPV16 prevalence was highest at 30.8 percent, followed by HPV6 at 6.7 percent and HPV18 at 6.6 percent (17). A large case series analyzing HPV positivity showed 70 to 100 percent of penile intraepithelial neoplasia cases were HPV positive, whereas only 40 to 50 percent of invasive penile cancer cases were HPV positive, suggesting alternative pathways for development of invasive disease (14). Prevention of HPV infection is of utmost importance, and the Gardasil 9 valent vaccination is now widely available, protective against 9 of the most commonly found strains.
Cost
Due to the rarity of the disease, there is little data on the cost of penile cancer treatment. Keeping et al. reported on the annual economic burden of penile cancer treatment between 2006 and 2011 using European Association of Urology penile cancer treatment guidelines. Their analysis of mean annual cost of treating invasive penile cancer was £3,737 per inpatient and £1,051 per outpatient, total mean annual costs of £2,442,020, and follow up and full treatment course averages between £7,421 and £8,063. The latter was consistent with estimated treatment costs for bladder and prostate cancers (18,19). Although it is a rare disease, the economic burden of treatment is significant, speaking to the importance of education, patient awareness and prevention.
Non-surgical treatment modalities
Non-surgical treatment modalities for penile cancer include topical medications, laser ablation and radiotherapy which should be reserved for non-invasive disease including carcinoma in situ (CIS), which is comprised of clinical variants, including Bowenoid papulosis, Erythroplasia of Queyrat, Bowen Disease (20), with Ta and selective T1 tumors (21). Penile tissue preservation is important for psychological well-being and sexual function in penile cancer (22). These non-surgical therapies are reserved for superficial lesions. Invasive disease should be treated with surgical excision.
Topical therapies
Topical therapies are a mainstay of initial therapy for CIS, including topical chemotherapy with 5-fluorouracil (5-FU) 5 percent cream and immunomodulation with imiquimod (IQ) 5 percent cream as first- and second-line therapies, respectively. Topical therapy is appealing because it is cost effective and is easily delivered in an ambulatory setting with generally tolerable local side effects (23). Alnajjar and others used topical 5-FU as treatment of CIS of the penis in 44 men and achieved a 57 percent complete response (CR) and a 13.6 percent partial response, with overall 70.6 percent response rate, on 34-month follow up (23). In 1976, Goette treated 7 men with erythroplasia of Queyrat successfully using 5-FU with no recurrences on 70 month follow-up, establishing 5-FU as an effective treatment (24). Data supporting IQ use is mainly from case reports and case series. Most treatment schedules are 5 times weekly for 4 to 6 weeks (22). Deen and others performed review of available literature pertaining to topical IQ therapy for CIS of the penis. The regimens varied widely from one to three times per week, with treatment course ranging from 11 days to 24 months. They found amongst all studies a CR of 63 percent, comprising 30 of 48 patients, partial response of 8 percent, which consisted of 4 patients, and no response in 29 percent, or14 patients (25). Due to the heterogeneity of studies using IQ, it is difficult to draw firm conclusions about its efficacy.
Photodynamic therapy
Photodynamic therapy has been used successfully in a case series of 11 patients with 100 percent response rate, with CR in 9 of 11 patients and partial response in the other two patients (26).
Laser therapy
Laser therapies include neodymium: yttrium aluminium garnet (Nd:YAG) laser coagulation and carbon dioxide (CO2) laser vaporization. The carbon dioxide laser has fallen into favor in recent years over the Nd:YAG because of its more superficial effect on penile tissue. The Nd:YAG laser penetrates to 3–4 mm, causing generally larger cosmetic defects and less effective cell death at the surface level (27).
For either modality, laser excision of the lesion is performed followed by tumor bed coagulation with a 3–5 mm margin of healthy tissue in pT1 patients and 0.5 to 1 centimeter margin in pT2 patients. Tewari and others successfully treated 32 patients—pT1 in 25 and pT2 in 7 patients—with Nd:YAG laser coagulation of penile cancer with good post treatment cosmesis, penile tissue preservation, and with micturition in the standing position preserved in all patients. There were no deaths, and two patients progressed after laser treatment requiring more invasive therapies (27). In one series, 19 patients with CIS of the penis were treated from 1986 to 2000 with either carbon dioxide laser or Nd:YAG laser with success and with at least 5 year follow up. All patients achieved a CR with 5 recurrences within 5 years that were successfully treated with another round of the CO2 laser vaporization. Only 1 patient progressed to invasive SCC requiring penectomy and node dissection (28).
The pulsed-dye laser is a newer non-ablative laser that has shown efficacy in 5 patients with CIS over a 2-year period through photothermal and phototoxic effects on cancer cells. All 5 patients achieved a CR, confirmed by control biopsy in 4 out of 5, with minimal to no residual scarring or hyperpigmentation and no recurrences on follow up, with range 16 to 41 months (20).
Torelli et al. achieved a CR in 6/10 patients with HPV-positive CIS of the penis treated with topical imiquimod followed by carbon dioxide laser therapy. There were no relapses at 26-month follow up. Two of ten patients had stable disease, and two of ten had disease progression, ultimately requiring total penectomy. Neither of these patients had HPV-positive disease (29).
A word on margins
Traditionally, the recommended resection margin was 1 to 2 centimeters, but this has been challenged in recent years due to the trend toward less invasive approaches and tissue conservation for psychological health. Per the 2005 European guidelines, the generally accepted margin for penile cancer is 5 mm, especially for low-grade tumors (30).
In 2000, Agrawal and others examined 64 penile SCC tumors, noting maximum proximal histologic extension of no more than 5 mm for tumor grades 1 and 2 and no more than 10 mm for tumors grade 3, concluding 10 mm margin for grades 1 to 2 and 15 millimeter margin for grade 3 as adequate (31). Minhas and others published on 51 cases of penile cancer showing that the traditional 2 centimeter margin was unnecessarily large. In their series, 48 percent of the margins were measured within 10 millimeters from the tumor edge, and 90 percent of the margins were less than 20 millimeters from the tumor edge. On follow up, only 4 percent developed local tumor recurrence requiring additional surgery (32). Additionally, Philippou and others reported on 179 penile cancer patients, finding the distance between the excised margin and tumor to be between 0 to 5 millimeters in greater than 60 percent of cases. Smaller margins allowed for greater tissue preservation, and anyone with positive margin underwent further resection. At 5-year follow-up, the smaller margin did not jeopardize oncologic control with a disease specific survival of 91.7 percent for those with any local recurrences. They noted a need for aggressive tumor resection and strict follow up for patients with adverse pathologic features including lymphovascular invasion and higher tumor stage or grade (33). This vigilant approach is key for all practicing physicians treating penile cancer. Surgeons should maintain a high level of suspicion for tumor recurrence with close follow up and appropriate patient counseling on recurrence risk.
Moh’s surgery
Moh’s micrographic surgery (MMS), first introduced in 1985, is a penile-sparing technique typically used for low grade, distal lesions (34). It involves fresh frozen sections, reviewed intraoperatively by a surgeon or pathologist, until a negative microscopic margin is achieved. MMS allows for assured cancer eradication while preserving maximal tissue and function—issues of upmost importance in this cancer type. The technique requires nuanced expertise and is mostly performed in high volume centers with skilled specialists.
In the original 1985 study by Mohs et al., the 5-year overall cure rate was 68 percent, with 81 percent for distal lesions of the glans or prepuce, and 57 percent for shaft lesions. They noted excellent functional urinary and sexual outcomes (34). A follow up 5-year study showed local cure rate of 94 percent and 5-year overall cure rate of 74 percent (35). More recently, Shindel and others reviewed 33 patients with CIS to T3 disease, with 63 percent having CIS, undergoing MMS with average of 58-month follow-up (36). They noted 32 percent local recurrence rate, successfully treated with repeat MMS in seven of eight patients. Of the largest early case series, recurrence rates after MMS ranged from 26 to 32 percent (34,35,37), and traditionally, MMS has been used to treat low grade, distal penile tumors because of this.
Recent literature argues for MMS use for invasive penile SCC (38). Machan and others reported using MMS to treat 44 penile cancers ranging from CIS to invasive SCC, with an overall combined recurrence rate of 11.1 percent, much lower than previously reported. Of the ten patients with primary invasive SCC, there were no local recurrences. Their explanation for higher recurrence rates using MMS in penile cancer were twofold. The contoured anatomy of the penis and elasticity of the tissue poses an extra challenge for identifying positive margins. Also, HPV infections of cells are not unidentifiable to the naked eye or detectable histologically at time of MMS, and it is believed that these cells, while negative at the time of resection, undergo malignant transformation later (38). Complications of MMS typically involve glans disfigurement and urethral meatal stenosis (39).
While the landscape for its use for invasive SCC remains debatable, MMS provides high cure rates and tissue conservation as an alternative to partial or radical penectomy for low grade and distal tumors.
Glans resurfacing
Glans resurfacing (Figure 1) is a technique that first developed in the management of lichen sclerosis, but has since been adapted to the management of penile CIS (41). Resurfacing entails the removal of the glanular epithelium and the subepithelial tissue down to the underlying spongiosum, followed by its replacement with a graft, usually split thickness skin graft (STSG) (42). Total glans resurfacing is the typical strategy employed, in contrast to partial glans resurfacing. In total glans resurfacing, the glans is pre-marked in quadrants. Then, sharp dissection of the glans epithelium and subepithelial tissue peels the specimen off the corpus spongiosum, starting at the meatus and extending to the coronal sulcus of each quadrant, followed by deep biopsies of underlying spongiosum (40,43). Partial glans resurfacing, on the other hand, treats solitary CIS lesions comprising less than half the glans, with removal of only the local epithelial and subepithelial tissue of the concerning lesion, with clearly negative margins and accompanying peripheral and deep biopsies (40). In both total and partial glans resurfacing, the denuded glans is typically covered with STSG, usually from the thigh. Alternative graft materials have been reported for use in glans resurfacing for penile SCC, with one group reporting use of testicular tunica vaginalis with good graft take (44).
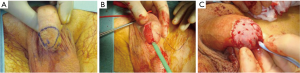
The need for an effective bridge between less invasive therapies like laser and topical therapy versus more radical approaches to achieve oncologic control while maximizing quality of life is important, as up to one-third of patients with penile CIS have underlying invasive SCC (45). The outcomes data for glans resurfacing seem to suggest it as a viable bridge for these patients with glanular CIS. One study demonstrated that total glans resurfacing has a 4.5 percent recurrence rate at a median 40-month follow up, while another smaller study demonstrated no recurrence with glans resurfacing at mean 15-month follow up (46,47).
A more recent British report on outcomes of both total and partial glans resurfacing in the setting of penile CIS noted overall recurrence rate of 4 percent over a 29-month period, though there was a 48 percent positive surgical margin rate, with 28 percent needing further surgery (40). Notwithstanding, the cosmetic results of glans resurfacing are noted to be similar to those achieved with laser therapy, with one study demonstrating close to 95 percent STSG complete take with median 5 out of 5 aesthetic scores (28,48). One study noted that all patients who were sexually active before total glans resurfacing became sexually active again by 6-month follow-up (49). When placed head-to-head against the more radical approach total glansectomy for CIS, a Swedish study of 27 patients demonstrated that glans resurfacing, like glansectomy, did not lead to disease recurrence at median 16-month follow up (50).
Partial penectomy, including glansectomy
Data suggest that up to 80 percent of penile SCC lesions occur distally at the glans or prepuce, and as such, patients with these locally confined lesions who have favorable stage and grade are considered good candidates for penile-sparing surgery (51). Specifically, the urologist should consider glansectomy for these limited lesions as opposed to more radical proximal amputation.
Advanced techniques for glanular reconstruction after glansectomy include using STSG. This technique is performed after glansectomy by first taking the remaining penile shaft skin, which is fixed to the underlying corporal bodies in a way that creates a shape similar to that of a glans from the exposed corporal heads, and leaving the distal 2 cm for placement and fixation of the STSG (Figure 2). The STSG is usually obtained from the thigh and tailored to fit the neo-glans, usually suturing the graft above Bucks fascia as opposed to below, unless oncologic control necessitates deeper dissection to the tunica albuginea. Graft take can be reduced in the setting of deeper dissection, likely due to the avascular quality of the tunica (52,53).
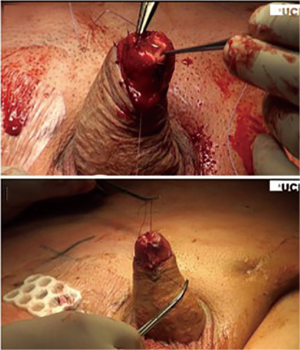
One group demonstrated that glansectomy with reconstruction using STSG had a 4 percent recurrence rate at 27-month mean follow up, with 51 percent of all patients in the study having T2 disease. The group also demonstrated that this penis-sparing technique can be applied in treating recurrence after prior radiation therapy, with good cosmetic and oncologic outcomes (54). Glansectomy can even be applied for patients with more advanced disease. A recent series studied patients with higher stage lesions who underwent glansectomy with reconstruction using STSG and found a local recurrence rate of 9 percent. This study’s cohort had a cancer-specific mortality of 11 percent over a median 41-month follow up, including patients with advanced disease, consisting of 56 percent T2 and 11 percent T3 disease (52). Of note, predictors of recurrence after glansectomy include perineural invasion, CIS, positive margins, and high grade SCC (55).
The role that glansectomy plays in the penile cancer management toolbox cannot be overstated. Patient shame from having a smaller penis size and absence of glans contributed to sexual abstinence in more than half of patients treated with partial penectomy in one study (56). However, penile amputation proximal to the glans cannot be avoided in certain cases, keeping in mind the aforementioned primary goal of oncologic control with the sometimes competing goal of quality of life: sexual and urinary function along with cosmesis.
Primary closure is an option preferred by some surgeons due its relative ease, involving directly suturing one end of the residual glans to the other, and often involving a conical appearance of the glans (57). This method has been described to have comparatively poor cosmetic and functional outcomes, with deformed and scarred appearance of the glans and dislocation of the urethral meatus with resultant urinary stream splitting and dribbling reported as complications (58).
The preputial flap is a viable option for glans reconstruction for superficial glans cancer that has been demonstrated to have better performance in orgasmic function, intercourse satisfaction, and overall satisfaction compared to primary closure (58). The procedure is performed by mobilizing adjacent shaft skin to cover small glanular defects. Furthermore, benefits over STSG reported by Yang and colleagues include increased sensitivity for orgasmic function due to preserved nerve endings, maximal subcuticular tissue to fill glanular defects, matching color of the flap to the glans compared to thigh or forearm donor sites, and the relative simplicity of having only one surgical site as opposed to having both the donor and recipient sites (58).
Covering the glanular defect with a urethral flap has also been described as a good option. Belinky and colleagues describe a one-stage technique of mobilizing the pendulous urethra down to the penoscrotal junction, spatulating the ventral end, and taking an approximately 2 centimeter distal segment to advance over and cover the distal tips of the corpora cavernosa. In a small study, their group described no neomeatal stenosis or flap necrosis, but there was a 10 percent rate of ventral curvature, which did not affect penetration for intercourse (59).
Though buccal mucosal graft has been reported to be used as a graft material to cover these glanular defects, authors like Palminteri and colleagues prefer not to use it due its better application in moist, not dry, environments. They cited findings of graft desquamation in some of their patients for which the buccal graft was used in two-stage resurfacing (60).
Numerous techniques have been described as adjuncts to partial penectomy to prevent the appearance of the residual penis as a stump to optimize both cosmetic and functional outcomes, in an effort to avoid the need for total penectomy for reasons of short stump length alone. These techniques include suprapubic lipectomy, penile suspensory ligament division, augmentation corporoplasty with incisions and grafting of the corpora, and ventral phalloplasty (61). Ventral phalloplasty in particular has been offered by Carrion’s group first as an option to maximize patients’ perception of penile length in the setting of prosthetic surgery, and then in the setting of partial penectomy for malignancy. The technique is performed by delineating and excising the redundant skin of the penoscrotal web and then longitudinally reapproximating the incision in a Heineke-Mikulicz fashion to reconstruct the raphe (62).
Total penectomy
Total penectomy may not be avoidable if the patient has high grade T1 disease, T2 or greater disease, or if partial penectomy leaves a penile stump that is too short (1). In this context, organ-sparing obviously cannot be achieved, but some advances in reconstructive techniques have provided improved quality of life for the men afflicted by these advanced forms of penile SCC (57).
Numerous advances in the arena of total penile reconstruction after amputation have been made since the first reports of it in practice at the beginning of the last century with the use of abdominal wall pedicles (63). These initial cases were often done for traumatic amputation, but the field developed further in the setting of female-to-male gender reassignment surgery. These advances translated to application for reconstruction after penectomy for SCC. The newer approaches’ success has been predicated on the emergence of reliable operative microscopy and associated techniques, employing staged strategies with use of grafts and rotational flaps (64,65). Specifically, the most common technique for neophalloplasty employed in modern times is the radial artery free flap (RAFF), with the second most common technique being the anterolateral thigh flap. There have been multiple other methods reported, using tissue from the scapula or lattisimus dorsi, thoracodorsal artery free flap, fibula, and even rotational flaps from the abdomen, groin, and thigh (66-68).
RAFF (Figure 3A,B) requires dissection of the hairless medial aspect of the forearm to be used for the neo-urethra, which is tubularized over a catheter. This tube is wrapped by another flap to create a tube-within-a-tube configuration. A neo-glans is created with a skin flap or graft. This neo-phallus is then anastomosed using the microscope, conventionally requiring a good connection of the urethra, radial to femoral artery, cephalic to saphenous vein, and antebrachial to ilioinguinal nerve. The placement of a penile prosthesis and further sculpting of the glans is usually performed in a second stage (71-73). In a study of 287 patients, 41 percent had urologic complications. Of these patients, 72 had fistula, most of which closed spontaneously, and 21 had urethral strictures, most of which required secondary or tertiary urethroplasties (69).
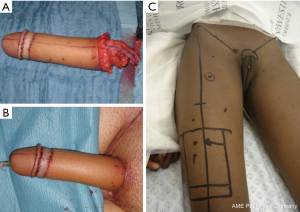
An alternative to the RAFF reported by some is the thigh flap. The anterolateral thigh flap is taken with anterolateral thigh perforators, preserving femoral nerve branches, and then tubularized, passed deep to the rectus femoris and sartorius and then through a subcutaneous tunnel to the perineum (Figure 3C). One of the posited benefits of the thigh flap over RAFF and other techniques is the more likely pigmentation color match with the perineum. The defect at the donor site requires a STSG (70).
One-stage radial and fibial osteocutaneous flaps are also often used in penile reconstruction, with the central radial or fibial bones being used to maintain rigidity for intercourse (74). However, there have been reports of decreased penile rigidity with time, with the neophallus often not being rigid enough for sexual intercourse. Some reasons for this discrepancy include soft tissue resorption and bone resorption over time. Kim’s group applied numerous corrective measures with some success, using techniques such as fat injection, artificial dermis grafting, silicone rod insertion, or autogenous rib bone cartilaginous graft for phallic tip augmentation (75).
Both semirigid and inflatable penile prostheses are sometimes placed to provide rigidity to the neophallus and allow for the patient to engage in penetrative intercourse. However, these implantations are inherently complicated due to lack of native tunica or corpora that would have otherwise provided protection from extrusion, infection, and mechanical dysfunction (76). Infection rate was cited at 12 percent, with 8 percent extrusion rate, in the literature for female-to-male transgender patients who received an implant after neophallus creation (77).
Zuckerman’s group reviewed outcomes in patients who received a penile prosthesis at an average of over 4.5 years after initial neophallus reconstruction, with implants placed via bilateral incisions over the ischial tuberosities. The reported explantation or revision rate in his series of 31 patients receiving either inflatable or semirigid penile prostheses was 23 percent, with postoperative complications including infection and erosion, and over 80 percent of patients were sexually active after prosthesis placement (78). A study of 130 patients who underwent RAFF followed by implant placement showed that 45 percent of these patients required revision or explantation surgery (69). It is suggested to wait at least a year after RAFF neophalloplasty to place the penile implant due to improved sensitivity (79). Advances in the development of better prosthetics in general understandably inspire further innovation in the use of implants in the neophalloplasty setting.
Penile transplant
The penile transplant is a technique that has found some limited utility, with recent reports of successful transplants in the US and elsewhere. The South African group of van der Merwe and colleagues first reported a successful cadaveric penile transplant with good functional and cosmetic outcomes. This first case required a 9-hour surgery, requiring two reinterventions, one for arterial thrombus and another for infected hematoma and proximal skin necrosis. The recipient reported satisfactory sexual intercourse 5 weeks after the transplant (80). Cetrulo’s group describe the first successful penile transplant in the US, with amputation of the donor allograft at the pubic bone to maximize corporal and urethral length with bilateral fasciocutaneous flaps harvested with external pudendal vessels. Microscopic anastomoses were performed. Reintervention was required twice for hematoma evacuation and eschar debridement, but ultimately the patient achieved satisfactory functional and cosmetic outcomes (81).
Tissue engineering
The field of tissue engineering has provided some inspiration for neophallic reconstruction that could be applied in the future. Numerous groups have investigated growing corporal smooth muscle cells into structures resembling the corpora that could potentially be used as penile substitutes, employing scaffolds such as polyglycolic acid and decellularized cadaveric corpora, with some success in animal studies (82). Cell sheet tissue engineering was used to create bioengineered urethras, with adipose-derived stem cells, oral mucosal epithelial cells, and oral mucosal fibroblasts tubularized into 3-centimeter segments with good long-term viability in a canine model (83). These advances in the field are a few examples of the potential to create new phalluses more akin to the native organs lost at penectomy than the currently available alternatives.
Conclusions
There is a spectrum of potential loss of penile tissue in the treatment of the potentially devastating diagnosis of penile SCC, ranging from small excisions of the glanular tumors to total penectomy. Yet advances in the field of penile reconstruction have answered the need for a corresponding spectrum of options to optimize both functional and cosmetic outcomes. As the field continues to evolve in the years to come, providers and patients can lean on hope in a better future for management of this disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Philippe E. Spiess) for the series “Rare Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2019.11.04). The series “Rare Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clark PE, Spiess PE, Agarwal N, et al. Penile Cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2013;11:594-615. [Crossref] [PubMed]
- Djajadiningrat RS, van Werkhoven E, Meinhardt W, et al. Penile Sparing Surgery for Penile Cancer—Does it Affect Survival? J Urol 2014;192:120-5. [Crossref] [PubMed]
- Lindegaard JC, Nielsen OS, Lundbeck FA, et al. A retrospective analysis of 82 cases of cancer of the penis. Br J Urol 1996;77:883-90. [Crossref] [PubMed]
- Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: A systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol 2009;9:8. [Crossref] [PubMed]
- Bleeker MC, Heideman DA, Snijders PJ, et al. Penile cancer: epidemiology, pathogenesis and prevention. World J Urol 2009;27:141-50. [Crossref] [PubMed]
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev 2007;16:1833-9. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, et al. Incidence trends in primary malignant penile cancer. Urol Oncol 2007;25:361-7. [Crossref] [PubMed]
- Hansen BT, Orumaa M, Lie AK, et al. Trends in incidence, mortality and survival of penile squamous cell carcinoma in Norway 1956-2015. Int J Cancer 2018;142:1586-93. [Crossref] [PubMed]
- Misra S, Chaturvedi A, Misra NC. Penile carcinoma: a challenge for the developing world. Lancet Oncol 2004;5:240-7. [Crossref] [PubMed]
- Pukkala E, Weiderpass E. Socio-economic differences in incidence rates of cancers of the male genital organs in Finland, 1971-95. Int J Cancer 2002;102:643-8. [Crossref] [PubMed]
- Maden C, Sherman KJ, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 1993;85:19-24. [Crossref] [PubMed]
- Daling JR, Madeleine MM, Johnson LG, et al. Penile cancer: importance of circumcision, human papillomavirus and smoking in in situ and invasive disease. Int J Cancer 2005;116:606-16. [Crossref] [PubMed]
- Adashek JJ, Necchi A, Spiess PE. Updates in the molecular epidemiology and systemic approaches to penile cancer. Urol Oncol 2019;37:403-8. [Crossref] [PubMed]
- Dillner J, von Krogh G, Horenblas S, et al. Etiology of squamous cell carcinoma of the penis. Scand J Urol Nephrol Suppl 2000;189-93. [Crossref] [PubMed]
- Flaherty A, Kim T, Giuliano A, et al. Implications for human papillomavirus in penile cancer. Urol Oncol 2014;32:53.e1-8. [Crossref] [PubMed]
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006;24 Suppl 3:S3/11-25.
- Backes DM, Kurman RJ, Pimenta JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009;20:449-57. [Crossref] [PubMed]
- Keeping ST, Tempest MJ, Stephens SJ, et al. Penile cancer treatment costs in England. BMC Public Health 2015;15:1305. [Crossref] [PubMed]
- Sangar VK, Ragavan N, Matanhelia SS, et al. The economic consequences of prostate and bladder cancer in the UK. BJU Int 2005;95:59-63. [Crossref] [PubMed]
- Niederkorn A, Sadoghi B, Komericki P. Pulsed-dye laser therapy for carcinoma in situ of the penis. Br J Dermatol 2018;179:195-6. [Crossref] [PubMed]
- Baumgarten AS, Fisher JS, Lawindy SM, et al. Penile sparing surgical approaches for primary penile tumors: preserving function and appearance. Transl Androl Urol 2017;6:809-19. [Crossref] [PubMed]
- Manjunath A, Brenton T, Wylie S, et al. Topical Therapy for non-invasive penile cancer (Tis)-updated results and toxicity. Transl Androl Urol 2017;6:803-8. [Crossref] [PubMed]
- Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. Eur Urol 2012;62:923-8. [Crossref] [PubMed]
- Goette DK, Carson TE. Erythroplasia of Queyrat: treatment with topical 5-fluorouracil. Cancer 1976;38:1498-502. [Crossref] [PubMed]
- Deen K, Burdon-Jones D. Imiquimod in the treatment of penile intraepithelial neoplasia: An update. Australas J Dermatol 2017;58:86-92. [Crossref] [PubMed]
- Filonenko E, Kaprin A, Alekseev B, et al. Own experience in treatment of patients with penile cancer using photodynamic therapy. Biomed Res Int 2015;2015:245080 [Crossref] [PubMed]
- Tewari M, Kumar M, Shukla HS. Nd:YAG laser treatment of early stage carcinoma of the penis preserves form and function of penis. Asian J Surg 2007;30:126-30. [Crossref] [PubMed]
- van Bezooijen BP, Horenblas S, Meinhardt W, et al. Laser therapy for carcinoma in situ of the penis. J Urol 2001;166:1670-1. [Crossref] [PubMed]
- Torelli T, Catanzaro MA, Nicolai N, et al. Treatment of Carcinoma In Situ of the Glans Penis With Topical Imiquimod Followed by Carbon Dioxide Laser Excision. Clin Genitourin Cancer 2017;15:e483-7. [Crossref] [PubMed]
- Hakenberg OW, Compérat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- Agrawal A, Pai D, Ananthakrishnan N, et al. The histological extent of the local spread of carcinoma of the penis and its therapeutic implications. BJU Int 2000;85:299-301. [Crossref] [PubMed]
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005;96:1040-3. [Crossref] [PubMed]
- Philippou P, Shabbir M, Malone P, et al. Conservative Surgery for Squamous Cell Carcinoma of the Penis: Resection Margins and Long-Term Oncological Control. J Urol 2012;188:803-8. [Crossref] [PubMed]
- Mohs FE, Snow SN, Messing EM, et al. Microscopically controlled surgery in the treatment of carcinoma of the penis. J Urol 1985;133:961-6. [Crossref] [PubMed]
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am 1992;19:291-304. [PubMed]
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term follow up. J Urol 2007;178:1980-5. [Crossref] [PubMed]
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol 1987;13:1163-7. [Crossref] [PubMed]
- Machan M, Brodland D, Zitelli J. Penile Squamous Cell Carcinoma: Penis-Preserving Treatment With Mohs Micrographic Surgery. Dermatol Surg 2016;42:936-44. [Crossref] [PubMed]
- Kamel MH, Bissada N, Warford R, et al. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J Urol 2017;198:770-9. [Crossref] [PubMed]
- Shabbir M, Muneer A, Kalsi J, et al. Glans Resurfacing for the Treatment of Carcinoma In Situ of the Penis : Surgical Technique and Outcomes. Eur Urol 2011;59:142-7. [Crossref] [PubMed]
- Depasquale I, Park AJ, Bracka A. The treatment of balanitis xerotica obliterans. BJU Int 2000;86:459-65. [Crossref] [PubMed]
- Sharma P, Zargar-Shoshtari K, Spiess PE. Current surgical management of penile cancer. Curr Probl Cancer 2015;39:147-57. [Crossref] [PubMed]
- Sosnowski R, Kuligowski M, Kuczkiewicz O, et al. Primary penile cancer organ sparing treatment. Cent European J Urol 2016;69:377-83. [PubMed]
- Weibl P, Plank C, Hoelzel R, et al. Neo-glans reconstruction for penile cancer : Description of the primary technique using autologous testicular tunica vaginalis graft. Arab J Urol 2018;16:218-23. [Crossref] [PubMed]
- Mikhail GR. Cancers, precancers, and pseudocancers on the male genitalia. A review of clinical appearances, histopathology, and management. J Dermatol Surg Oncol 1980;6:1027-35. [Crossref] [PubMed]
- Chipollini J, Yan S, Ottenhof SR, et al. Surgical management of penile carcinoma in situ: results from an international collaborative study and review of the literature. BJU Int 2018;121:393-8. [Crossref] [PubMed]
- Pérez-Niño J, Fernandez N, Sarmiento G. Partial penectomy and penile reconstruction. Initial surgical management of localized penile cancer. Actas Urol Esp 2014;38:62-5. [PubMed]
- O’Kelly F, Lonergan P, Lundon D, et al. A Prospective Study of Total Glans Resurfacing for Localized Penile Cancer to Maximize Oncologic and Functional Outcomes in a Tertiary Referral Network. J Urol 2017;197:1258-63. [Crossref] [PubMed]
- Fenner A. Penile cancer : Total glans resurfacing viable for all. Nat Rev Urol 2017;14:198. [Crossref] [PubMed]
- Håkansson U, Kirrander P, Uvelius B, et al. Organ-sparing reconstructive surgery in penile cancer: initial experiences at two Swedish referral centres. Scand J Urol 2015;49:149-54. [Crossref] [PubMed]
- Martins FE, Rodrigues RN, Lopes TM. Organ-Preserving Surgery for Penile Carcinoma. Adv Urol 2008;2008:634216 [Crossref] [PubMed]
- Parnham AS, Albersen M, Sahdev V, et al. Surgery in Motion Glansectomy and Split-thickness Skin Graft for Penile Cancer. Eur Urol 2018;73:284-9. [Crossref] [PubMed]
- Parnham AS, Albersen M, Sahdev V, et al. Glansectomy and Split-thickness Skin Graft for Penile Cancer. Eur Urol 2018;73:284-9. [Crossref] [PubMed]
- Smith Y, Hadway P, Biedrzycki O, et al. Reconstructive Surgery for Invasive Squamous Carcinoma of the Glans Penis. Eur Urol 2007;52:1179-85. [Crossref] [PubMed]
- Albersen M, Ph D, Parnham A, et al. Predictive factors for local recurrence after glansectomy and neoglans reconstruction for penile squamous cell carcinoma. Urol Oncol 2018;36:141-6. [Crossref] [PubMed]
- Romero FR. Sexual function after partial penectomy for penile cancer. Urology 2005;66:1292-5. [Crossref] [PubMed]
- Burnett AL. Penile preserving and reconstructive surgery in the management of penile cancer. Nat Rev Urol 2016;13:249-57. [Crossref] [PubMed]
- Yang J, Chen J, Wu XF, et al. Glans-reconstruction with preputial flap is superior to primary closure for post-surgical restoration of male sexual function in glans-preserving surgery. Andrology 2014;2:729-33. [Crossref] [PubMed]
- Belinky JJ, Cheliz GM, Graziano CA, et al. Glanuloplasty with urethral flap after partial penectomy. J Urol 2011;185:204-6. [Crossref] [PubMed]
- Palminteri E, Berdondini E, Lazzeri M, et al. Resurfacing and Reconstruction of the Glans Penis. Eur Urol 2007;52:893-8. [Crossref] [PubMed]
- Hakky TS, Rodriguez AR, Parker J, et al. Ventral Phalloplasty. Int Braz J Urol 2012;38:565. [Crossref] [PubMed]
- Wallen JJ, Baumgarten AS, Kim T, et al. Optimizing penile length in patients undergoing partial penectomy for penile cancer: Novel application of the ventral phalloplasty oncoplastic technique. Int Braz J Urol 2014;40:708. [Crossref] [PubMed]
- Schultheiss D, Gabouev AI, Jonas U. Bogoraz (1874-1952): pioneer of phalloplasty and penile implant surgery. J Sex Med 2005;2:139-46. [Crossref] [PubMed]
- Hage JJ, Bloem JJ, Suliman HM. Review of the literature on tech- niques for phalloplasty with emphasis on the applicability in female-to-male transsexuals. J Urol 1993;150:1093-8. [Crossref] [PubMed]
- Hage JJ, De Graaf FH. Addressing the ideal requirements by free flap phalloplasty: some reflections on refinements of technique. Microsurgery 1993;14:592-8. [Crossref] [PubMed]
- Garaffa G, Antonini G, Gentile V, et al. Phalloplasty for the genetic male. Transl Androl Urol 2012;1:103-8. [PubMed]
- Sopko NA, Tuffaha SH, Lough D, et al. Penile allotransplantation for complex genitourinary reconstruction. J Urol 2017;198:274-80. [Crossref] [PubMed]
- Lin CT, Chen LW. Using a free thoracodorsal artery perforator flap for phallic reconstruction - A report of surgical technique. J Plast Reconstr Aesthet Surg 2009;62:402-8. [Crossref] [PubMed]
- Monstrey S, Hoebeke P, Selvaggi G, et al. Penile reconstruction: Is the radial forearm flap really the standard technique? Plast Reconstr Surg 2009;124:510-8. [Crossref] [PubMed]
- Descamps MJL, Hayes PM, Hudson DA. Phalloplasty in complete aphallia: pedicled anterolateral thigh flap. J Plast Reconstr Aesthet Surg 2009;62:e51-4. [Crossref] [PubMed]
- Garaffa G, Raheem AA, Christopher NA, et al. Total phallic reconstruction after penile amputation for carcinoma. BJU Int 2009;104:852-6. [Crossref] [PubMed]
- Dabernig J, Shelley OP, Cuccia G, et al. Urethral reconstruction using the radial forearm free flap: experience in oncologic cases and gender reassignment. Eur Urol 2007;52:547-53. [Crossref] [PubMed]
- Chang TS, Hwang WY. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg 1984;74:251-8. [Crossref] [PubMed]
- Kim SK, Lee KC, Kwon YS, et al. Phalloplasty using radial forearm osteocutaneous free flaps in female-to-male transsexuals. J Plast Reconstr Aesthet Surg 2009;62:309-17. [Crossref] [PubMed]
- Kim SK, Kim TH, Yang JI, et al. The etiology and treatment of the softened phallus after the radial forearm osteocutaneous free flap phalloplasty. Arch Plast Surg 2012;39:390-6. [Crossref] [PubMed]
- Bickell M, Beilan J, Wallen J, et al. Advances in Surgical Reconstructive Techniques in the Management of Penile, Urethral, and Scrotal Cancer. Urol Clin North Am 2016;43:545-59. [Crossref] [PubMed]
- Hoebeke PB, Decaestecker K, Beysens M, et al. Erectile Implants in Female-to-Male Transsexuals: Our Experience in 129 Patients. Eur Urol 2010;57:334-40. [Crossref] [PubMed]
- Zuckerman JM, Smentkowski K, Gilbert D, et al. Penile Prosthesis Implantation in Patients with a History of Total Phallic Construction. J Sex Med 2015;12:2485-91. [Crossref] [PubMed]
- Frey JD, Poudrier G, Chiodo MV, et al. An Update on Genital Reconstruction Options for the Female-To-Male Transgender Patient: A Review of the Literature. Plast Reconstr Surg 2017;139:728-37. [Crossref] [PubMed]
- van der Merwe A, Graewe F, Zühlke A, et al. Penile allotransplantation for penis amputation following ritual circumcision: a case report with 24 months of follow-up. Lancet 2017;390:1038-47. [Crossref] [PubMed]
- Cetrulo CL, Li ÃK, Salinas HM, et al. Penis Transplantation. Ann Surg 2018;267:983-8. [Crossref] [PubMed]
- Chouhan JD, Thakker PU, Terlecki RP. Engineering of erectile tissue: the state and future of corporal restoration. World J Urol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Zhou S, Yang R, Zou Q, et al. Fabrication of tissue-engineered bionic urethra using cell sheet technology and labeling by ultrasmall superparamagnetic iron oxide for full-thickness urethral reconstruction. Theranostics 2017;7:2509-23. [Crossref] [PubMed]
Cite this article as: Manimala NJ, Nealon SW, Heinsimer KR, Wiegand LR. Advances in penile reconstructive techniques for primary penile tumors. AME Med J 2019;4:43.

